Cortical sensorimotor alterations classify clinical phenotype and putative genotype of spasmodic dysphonia
- PMID: 27346568
- PMCID: PMC5308055
- DOI: 10.1111/ene.13067
Cortical sensorimotor alterations classify clinical phenotype and putative genotype of spasmodic dysphonia
Abstract
Background and purpose: Spasmodic dysphonia (SD), or laryngeal dystonia, is a task-specific isolated focal dystonia of unknown causes and pathophysiology. Although functional and structural abnormalities have been described in this disorder, the influence of its different clinical phenotypes and genotypes remains scant, making it difficult to explain SD pathophysiology and to identify potential biomarkers.
Methods: We used a combination of independent component analysis and linear discriminant analysis of resting-state functional magnetic resonance imaging data to investigate brain organization in different SD phenotypes (abductor versus adductor type) and putative genotypes (familial versus sporadic cases) and to characterize neural markers for genotype/phenotype categorization.
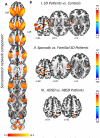
Results: We found abnormal functional connectivity within sensorimotor and frontoparietal networks in patients with SD compared with healthy individuals as well as phenotype- and genotype-distinct alterations of these networks, involving primary somatosensory, premotor and parietal cortices. The linear discriminant analysis achieved 71% accuracy classifying SD and healthy individuals using connectivity measures in the left inferior parietal and sensorimotor cortices. When categorizing between different forms of SD, the combination of measures from the left inferior parietal, premotor and right sensorimotor cortices achieved 81% discriminatory power between familial and sporadic SD cases, whereas the combination of measures from the right superior parietal, primary somatosensory and premotor cortices led to 71% accuracy in the classification of adductor and abductor SD forms.
Conclusions: Our findings present the first effort to identify and categorize isolated focal dystonia based on its brain functional connectivity profile, which may have a potential impact on the future development of biomarkers for this rare disorder.
Keywords: dystonia; imaging marker; resting-state networks.
© 2016 EAN.
Figures

References
-
- Blitzer A. Spasmodic dysphonia and botulinum toxin: experience from the largest treatment series. Eur J Neurol. 2010;17(Suppl 1):28–30. - PubMed
-
- Brin MF, Blitzer A, Stewart C. Laryngeal dystonia (spasmodic dysphonia): observations of 901 patients and treatment with botulinum toxin. Adv Neurol. 1998;78:237–252. - PubMed
-
- Ali SO, Thomassen M, Schulz GM, et al. Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: an H215O PET study. Journal of speech, language, and hearing research : JSLHR. 2006;49:1127–1146. - PubMed
-
- Haslinger B, Erhard P, Dresel C, Castrop F, Roettinger M, Ceballos-Baumann AO. "Silent event-related" fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology. 2005;65:1562–1569. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

