Circulating Tumor Cell Isolation and Analysis
- PMID: 27346614
- PMCID: PMC5123699
- DOI: 10.1016/bs.acc.2016.03.003
Circulating Tumor Cell Isolation and Analysis
Abstract
Isolation and analysis of cancer cells from body fluids have significant implications in diagnosis and therapeutic treatment of cancers. Circulating tumor cells (CTCs) are cancer cells circulating in the peripheral blood or spreading iatrogenically into blood vessels, which is an early step in the cascade of events leading to cancer metastasis. Therefore, CTCs can be used for diagnosing for therapeutic treatment, prognosing a given anticancer intervention, and estimating the risk of metastatic relapse. However, isolation of CTCs is a significant technological challenge due to their rarity and low recovery rate using traditional purification techniques. Recently microfluidic devices represent a promising platform for isolating cancer cells with high efficiency in processing complex cellular fluids, with simplicity, sensitivity, and throughput. This review summarizes recent methods of CTC isolation and analysis, as well as their applications in clinical studies.
Keywords: Biomarkers; Cancer; Circulating tumor cells; Diagnosis; Microfluidics.
© 2016 Elsevier Inc. All rights reserved.
Figures


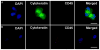
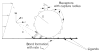
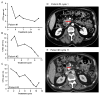
References
-
- Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8(5):329–340. - PubMed
-
- Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146–147.
-
- Allard WJ, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–6904. - PubMed
-
- Christopherson WM. Cancer cells in the peripheral blood: a second look. Acta Cytol. 1965;9:169–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

