Seven Post-synthetic Covalent Reactions in Tandem Leading to Enzyme-like Complexity within Metal-Organic Framework Crystals
- PMID: 27346625
- PMCID: PMC5376101
- DOI: 10.1021/jacs.6b04204
Seven Post-synthetic Covalent Reactions in Tandem Leading to Enzyme-like Complexity within Metal-Organic Framework Crystals
Abstract
The design of enzyme-like complexity within metal-organic frameworks (MOFs) requires multiple reactions to be performed on a MOF crystal without losing access to its interior. Here, we show that seven post-synthetic reactions can be successfully achieved within the pores of a multivariate MOF, MTV-IRMOF-74-III, to covalently incorporate tripeptides that resemble the active sites of enzymes in their spatial arrangement and compositional heterogeneity. These reactions build up H2N-Pro-Gly-Ala-CONHL and H2N-Cys-His-Asp-CONHL (where L = organic struts) amino acid sequences by covalently attaching them to the organic struts in the MOFs, without losing porosity or crystallinity. An enabling feature of this chemistry is that the primary amine functionality (-CH2NHBoc) of the original MOF is more reactive than the commonly examined aromatic amines (-NH2), and this allowed for the multi-step reactions to be carried out in tandem within the MOF. Preliminary findings indicate that the complexity thus achieved can affect reactions that were previously accomplished only in the presence of enzymes.
Figures


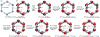
References
-
- Fracaroli AM, Furukawa H, Suzuki M, Dodd M, Okajima S, Gándara F, Reimer JA, Yaghi OM. J Am Chem Soc. 2014;136:8863. - PubMed
-
- Deng H, Grunder S, Cordova KE, Valente C, Furukawa H, Hmadeh M, Gándara F, Whalley AC, Liu Z, Asahina S, Kazumori H, O'Keeffe M, Terasaki O, Stoddart J, Yaghi OM. Science. 2012;336:1018. - PubMed
-
- Canivet J, Aguado S, Schuurman Y, Farrusseng D. J Am Chem Soc. 2013;135:4195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

