Limonene ozonolysis in the presence of nitric oxide: Gas-phase reaction products and yields
- PMID: 27346977
- PMCID: PMC4920481
- DOI: 10.1016/j.atmosenv.2016.03.003
Limonene ozonolysis in the presence of nitric oxide: Gas-phase reaction products and yields
Abstract
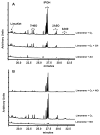
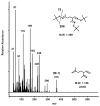
The reaction products from limonene ozonolysis were investigated using the new carbonyl derivatization agent, O-tert-butylhydroxylamine hydrochloride (TBOX). With ozone (O3) as the limiting reagent, five carbonyl compounds were detected. The yields of the carbonyl compounds are discussed with and without the presence of a hydroxyl radical (OH•) scavenger, giving insight into the influence secondary OH radicals have on limonene ozonolysis products. The observed reaction product yields for limonaketone (LimaKet), 7-hydroxyl-6-oxo-3-(prop-1-en-2-yl)heptanal (7H6O), and 2-acetyl-5-oxohexanal (2A5O) were unchanged suggesting OH• generated by the limonene + O3 reaction does not contribute to their formation. The molar yields of 3-isopropenyl-6-oxo-heptanal (IPOH) and 3-acetyl-6-oxoheptanal (3A6O) decreased by 68% and >95%; respectively, when OH• was removed. This suggests that OH• radicals significantly impact the formation of these products. Nitric oxide (NO) did not significantly affect the molar yields of limonaketone or IPOH. However, NO (20 ppb) considerably decreased the molar reaction product yields of 7H6O (62%), 2A5O (63%), and 3A6O (47%), suggesting NO reacted with peroxyl intermediates, generated during limonene ozonolysis, to form other carbonyls (not detected) or organic nitrates. These studies give insight into the transformation of limonene and its reaction products that can lead to indoor exposures.
Keywords: Derivatization; Oxygenated organic compounds; Ozone; Reaction products.
Figures

Similar articles
-
A new agent for derivatizing carbonyl species used to investigate limonene ozonolysis.Atmos Environ (1994). 2014 Dec;99:519-526. doi: 10.1016/j.atmosenv.2014.10.015. Atmos Environ (1994). 2014. PMID: 30100808 Free PMC article.
-
Gas-phase reaction products and yields of terpinolene with ozone and nitric oxide using a new derivatization agent.Atmos Environ (1994). 2015 Dec;122:520. doi: 10.1016/j.atmosenv.2015.10.015. Atmos Environ (1994). 2015. PMID: 31814795 Free PMC article.
-
Identification and quantification of carbonyl-containing α-pinene ozonolysis products using O-tert-butylhydroxylamine hydrochloride.J Atmos Chem. 2017 Sep;74(3):325-338. doi: 10.1007/s10874-016-9344-6. Epub 2016 Aug 26. J Atmos Chem. 2017. PMID: 28701805 Free PMC article.
-
Yields of carbonyl products from gas-phase reactions of fragrance compounds with OH radical and ozone.Environ Sci Technol. 2009 May 15;43(10):3561-8. doi: 10.1021/es803465v. Environ Sci Technol. 2009. PMID: 19544855
-
Use of denuder/filter apparatus to investigate terpene ozonolysis.J Environ Monit. 2012 Mar;14(3):1044-54. doi: 10.1039/c2em10799f. Epub 2012 Feb 15. J Environ Monit. 2012. PMID: 22334151
Cited by
-
Hyperconjugation enhances electrophilic addition to monocyclic monoterpenes: a Fukui function perspective.J Mol Model. 2018 Sep 29;24(10):300. doi: 10.1007/s00894-018-3825-2. J Mol Model. 2018. PMID: 30269163
-
Towards sustainable additive manufacturing: The need for awareness of particle and vapor releases during polymer recycling, making filament, and fused filament fabrication 3-D printing.Resour Conserv Recycl. 2022 Jan;176:10.1016/j.resconrec.2021.105911. doi: 10.1016/j.resconrec.2021.105911. Resour Conserv Recycl. 2022. PMID: 35982992 Free PMC article.
-
Reactive indoor air chemistry and health-A workshop summary.Int J Hyg Environ Health. 2017 Nov;220(8):1222-1229. doi: 10.1016/j.ijheh.2017.09.009. Epub 2017 Sep 23. Int J Hyg Environ Health. 2017. PMID: 28964679 Free PMC article. Review.
-
Effects of 222 nm Germicidal Ultraviolet Light on Aerosol and VOC Formation from Limonene.ACS EST Air. 2024 May 4;1(7):725-733. doi: 10.1021/acsestair.4c00065. eCollection 2024 Jul 12. ACS EST Air. 2024. PMID: 39021671 Free PMC article.
-
A chamber study of alkyl nitrate production formed by terpene ozonolysis in the presence of NO and alkanes.Atmos Environ (1994). 2017 Dec;171:132-148. doi: 10.1016/j.atmosenv.2017.10.002. Atmos Environ (1994). 2017. PMID: 30792610 Free PMC article.
References
-
- Aschmann SM, Arey J, Atkinson R. OH radical formation from the gas-phase reactions of O3 with a series of terpenes. Atmos Environ. 2002;36:4347–4355.
-
- Atkinson R, Arey J. Atmospheric degradation of volatile organic compounds. Chem Rev. 2003;103:4605–4638. - PubMed
-
- Atkinson R, Aschmann SM, Arey J, Shorees B. Formation of OH radicals in the gas phase reactions of O3 with a series of terpenes. J Geophys Res. 1992;97:6065–6073.
-
- Carslaw N. A mechanistic study of limonene oxidation products and pathways following cleaning activities. Atmos Environ. 2013;80:507–513.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
