Clinical manufacturing of CAR T cells: foundation of a promising therapy
- PMID: 27347557
- PMCID: PMC4909095
- DOI: 10.1038/mto.2016.15
Clinical manufacturing of CAR T cells: foundation of a promising therapy
Abstract
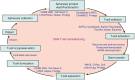
The treatment of cancer patients with autologous T cells expressing a chimeric antigen receptor (CAR) is one of the most promising adoptive cellular therapy approaches. Reproducible manufacturing of high-quality, clinical-grade CAR-T cell products is a prerequisite for the wide application of this technology. Product quality needs to be built-in within every step of the manufacturing process. We summarize herein the requirements and logistics to be considered, as well as the state of the art manufacturing platforms available. CAR-T cell therapy may be on the verge of becoming standard of care for a few clinical indications. Yet, many challenges pertaining to manufacturing standardization and product characterization remain to be overcome in order to achieve broad usage and eventual commercialization of this therapeutic modality.
Figures
References
-
- Yee, C (2014). The use of endogenous T cells for adoptive transfer. Immunol Rev 257: 250–263. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources