Loss of protein association causes cardiolipin degradation in Barth syndrome
- PMID: 27348092
- PMCID: PMC4955704
- DOI: 10.1038/nchembio.2113
Loss of protein association causes cardiolipin degradation in Barth syndrome
Abstract
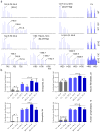
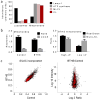
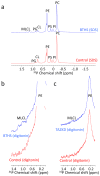
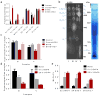
Cardiolipin is a specific mitochondrial phospholipid that has a high affinity for proteins and that stabilizes the assembly of supercomplexes involved in oxidative phosphorylation. We found that sequestration of cardiolipin in protein complexes is critical to protect it from degradation. The turnover of cardiolipin is slower by almost an order of magnitude than the turnover of other phospholipids. However, in subjects with Barth syndrome, cardiolipin is rapidly degraded via the intermediate monolyso-cardiolipin. Treatments that induce supercomplex assembly decrease the turnover of cardiolipin and the concentration of monolyso-cardiolipin, whereas dissociation of supercomplexes has the opposite effect. Our data suggest that cardiolipin is uniquely protected from normal lipid turnover by its association with proteins, but this association is compromised in subjects with Barth syndrome, leading cardiolipin to become unstable, which in turn causes the accumulation of monolyso-cardiolipin.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures

References
-
- Ren M, Phoon CKL, Schlame M. Metabolism and function of mitochondrial cardiolipin. Progr Lipid Res. 2014;55:1–16. - PubMed
-
- Lewis RNAH, McElhaney RN. The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim Biophys Acta. 2009;1788:2069–2079. - PubMed
-
- Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem. 2002;277:43553–43556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

