Eicosapentaenoic acid ameliorates hyperglycemia in high-fat diet-sensitive diabetes mice in conjunction with restoration of hypoadiponectinemia
- PMID: 27348201
- PMCID: PMC4931313
- DOI: 10.1038/nutd.2016.21
Eicosapentaenoic acid ameliorates hyperglycemia in high-fat diet-sensitive diabetes mice in conjunction with restoration of hypoadiponectinemia
Abstract
Background/objective: Eicosapentaenoic acid (EPA) exerts pleiotropic effects on metabolic disorders such as atherosclerosis and dyslipidemia, but its effectiveness in the treatment of type 2 diabetes mellitus remains controversial.
Methods: We examined the antidiabetic effect of EPA in insulin receptor mutant (Insr(P1195L/+)) mice that exhibit high-fat diet (HFD)-dependent hyperglycemia.
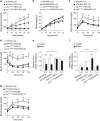
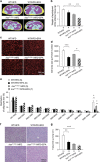
Results: EPA supplementation was found to alleviate hyperglycemia of Insr(P1195L/+) mice fed HFD (Insr(P1195L/+)/HFD mice), which was accompanied by amelioration of increased gluconeogenesis and impaired insulin signaling, as assessed by glucose-6-phosphatase (G6pc) expression on refeeding and insulin-induced phosphorylation of Akt in the liver, respectively. We found that serum levels of adiponectin, the antidiabetic adipokine, were decreased by HFD along with the body weight gain in Insr(P1195L/+) mice but not in wild-type mice, suggesting that Insr(P1195L/+) mice are prone to hypoadiponectinemia in response to obesity. Interestingly, the blood glucose levels of Insr(P1195L/+) mice were in reverse proportion to their serum adiponectin levels and EPA supplementation ameliorated their hyperglycemia in conjunction with the restoration of hypoadiponectinemia.
Conclusions: EPA exerts an antidiabetic effect in Insr(P1195L/+)/HFD mice, an HFD-sensitive, insulin-resistant animal model, possibly through its action against hypoadiponectinemia.
Figures



References
-
- Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 2011; 378: 169–181. - PubMed
-
- Bhaswant M, Poudyal H, Brown L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. J Nutr Biochem 2015; 26: 571–584. - PubMed
-
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72: 219–246. - PubMed
-
- Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res 2003; 44: 479–486. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

