Rationale and Design of the Randomized Evaluation of Default Access to Palliative Services (REDAPS) Trial
- PMID: 27348271
- PMCID: PMC5059505
- DOI: 10.1513/AnnalsATS.201604-308OT
Rationale and Design of the Randomized Evaluation of Default Access to Palliative Services (REDAPS) Trial
Abstract
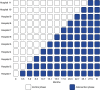
The substantial nationwide investment in inpatient palliative care services stems from their great promise to improve patient-centered outcomes and reduce costs. However, robust experimental evidence of these benefits is lacking. The Randomized Evaluation of Default Access to Palliative Services (REDAPS) study is a pragmatic, stepped-wedge, cluster randomized trial designed to test the efficacy and costs of specialized palliative care consultative services for hospitalized patients with advanced chronic obstructive pulmonary disease, dementia, or end-stage renal disease, as well as the overall effectiveness of ordering such services by default. Additional aims are to identify the types of services that are most beneficial and the types of patients most likely to benefit, including comparisons between ward and intensive care unit patients. We hypothesize that patient-centered outcomes can be improved without increasing costs by simply changing the default option for palliative care consultation from opt-in to opt-out for patients with life-limiting illnesses. Patients aged 65 years or older are enrolled at 11 hospitals using an integrated electronic health record. As a pragmatic trial designed to enroll between 12,000 and 15,000 patients, eligibility is determined using a validated, electronic health record-based algorithm, and all outcomes are captured via the electronic health record and billing systems data. The time at which each hospital transitions from control, opt-in palliative care consultation to intervention, opt-out consultation is randomly assigned. The primary outcome is a composite measure of in-hospital mortality and length of stay. Secondary outcomes include palliative care process measures and clinical and economic outcomes. Clinical trial registered with www.clinicaltrials.gov (NCT02505035).
Keywords: behavioral economics; electronic health records; palliative care; pragmatic clinical trial.
Figures

References
-
- Citizens’ Health Care Working Group. Interim recommendations of the Citizens’ Health Care Working Group. 2006 [accessed 2016 Jan 20]. Available from: http://govinfo.library.unt.edu/chc/interimrecs/interim_recommendations.html.
-
- Fields MJ, Cassel CK. Approaching death, improving care at the end of life. Washington, D.C: National Academy Press; 1997. - PubMed
-
- Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. - PubMed
-
- Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, Rubenfeld GD Robert Wood Johnson Foundation ICU End-Of-Life Peer Group. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638–643. - PubMed
-
- Kwok AC, Semel ME, Lipsitz SR, Bader AM, Barnato AE, Gawande AA, Jha AK. The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet. 2011;378:1408–1413. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

