Pretreatment serum lactate dehydrogenase is an independent prognostic factor for patients receiving neoadjuvant chemotherapy for locally advanced cervical cancer
- PMID: 27350066
- PMCID: PMC4971915
- DOI: 10.1002/cam4.779
Pretreatment serum lactate dehydrogenase is an independent prognostic factor for patients receiving neoadjuvant chemotherapy for locally advanced cervical cancer
Abstract
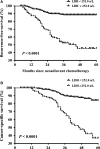
For locally advanced cervical cancer (LACC), hypoxia is a characteristic property. This study aimed to investigate whether baseline lactic dehydrogenase (LDH) level, which is a marker of hypoxia, had clinical value in determining neoadjuvant chemotherapy (NACT) response and prognosis for LACC patients. The study cohort included 418 patients with a median follow-up of 37.5 months. Cox proportional hazards models were used to assess the prognostic value of baseline LDH levels. Multivariate logistic regression analysis was performed to identify independent predictors of complete response after NACT. Backward stepwise selection with the Akaike information criterion was used to identify factors that could be entered into the multivariate regression model. Compared with patients with LDH levels <252.0 μ/L, patients with LDH levels ≥252.0 μ/L were more likely to have an elevated level of squamous cell carcinoma antigen, lymphatic vascular space involvement, lymph node metastasis, and positive parametrium and achieved lower complete remission rates. Baseline LDH levels ≥252.0 μ/L was an independent prognosticator for recurrence-free survival (adjusted hazard ratio [HR], 3.56; 95% confidence interval [CI] 2.22-5.69; P < 0.0001) and cancer-specific survival (adjusted HR, 3.08; 95% CI, 1.89-5.01; P < 0.0001). The predictive value of baseline LDH value remained significant in the subgroup analysis. LDH level ≥252.0 μ/L was identified as an independent predictor of complete remission after NACT (adjusted odds ratio [OR], 0.29; 95% CI, 0.15-0.58; P < 0.0001). Baseline LDH ≥252.0 μ/L is an independent prognostic predictor for patients receiving neoadjuvant chemotherapy for LACC. It helps distinguish patients with different prognosis and select patients who are more likely to benefit from NACT.
Keywords: Cervical cancer; lactate dehydrogenase; locally advanced cancer; prognosis.
© 2016 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Pretreatment glycemic control status is an independent prognostic factor for cervical cancer patients receiving neoadjuvant chemotherapy for locally advanced disease.BMC Cancer. 2017 Aug 3;17(1):517. doi: 10.1186/s12885-017-3510-3. BMC Cancer. 2017. PMID: 28774279 Free PMC article.
-
[Effects of pre-chemotherapy hemoglobin and platelet levels in patients with stage Ib2-IIb cervical cancer treated with neoadjuvant chemotherapy followed by radical hysterectomy].Zhonghua Fu Chan Ke Za Zhi. 2012 Aug;47(8):577-81. Zhonghua Fu Chan Ke Za Zhi. 2012. PMID: 23141176 Chinese.
-
Impact of Hyperglycemia on Outcomes among Patients Receiving Neoadjuvant Chemotherapy for Bulky Early Stage Cervical Cancer.PLoS One. 2016 Nov 16;11(11):e0166612. doi: 10.1371/journal.pone.0166612. eCollection 2016. PLoS One. 2016. PMID: 27851819 Free PMC article.
-
Successful neoadjuvant chemotherapy plus sintilimab for locally advanced cervical cancer: case series and review of the literature.Diagn Pathol. 2023 Sep 26;18(1):107. doi: 10.1186/s13000-023-01394-w. Diagn Pathol. 2023. PMID: 37752528 Free PMC article. Review.
-
Acquired treatment response from neoadjuvant chemotherapy predicts a favorable prognosis for local advanced cervical cancer: A meta-analysis.Medicine (Baltimore). 2018 Apr;97(17):e0530. doi: 10.1097/MD.0000000000010530. Medicine (Baltimore). 2018. PMID: 29703026 Free PMC article. Review.
Cited by
-
High Pretreatment LDH Predicts Poor Prognosis in Hypopharyngeal Cancer.Front Oncol. 2021 Mar 11;11:641682. doi: 10.3389/fonc.2021.641682. eCollection 2021. Front Oncol. 2021. PMID: 33777804 Free PMC article.
-
Association between serum lactate dehydrogenase and lymph node metastasis in cervical cancer.Oncol Lett. 2023 Sep 25;26(5):482. doi: 10.3892/ol.2023.14069. eCollection 2023 Nov. Oncol Lett. 2023. PMID: 37818132 Free PMC article.
-
A preoperative prediction of lymph node metastasis in early cervical squamous cell cancer with hematologica - based model.J Cancer. 2023 Jun 19;14(10):1763-1772. doi: 10.7150/jca.85301. eCollection 2023. J Cancer. 2023. PMID: 37476184 Free PMC article.
-
Prognostic Values of Systemic Inflammation Response (SIR) Parameters in Resectable Cervical Cancer.Dose Response. 2019 Feb 26;17(1):1559325819829543. doi: 10.1177/1559325819829543. eCollection 2019 Jan-Mar. Dose Response. 2019. PMID: 30833874 Free PMC article.
-
The novel pretreatment immune prognostic index discriminates survival outcomes in locally advanced non-operative esophageal squamous cell carcinoma patients treated with definitive chemoradiotherapy: a 6-year retrospective study.Transl Oncol. 2022 Jul;21:101430. doi: 10.1016/j.tranon.2022.101430. Epub 2022 Apr 19. Transl Oncol. 2022. PMID: 35452997 Free PMC article.
References
-
- Koh, W. J. , Greer B. E., Abu‐Rustum N. R., Apte S. M., Campos S. M., Cho K. R., et al. 2015. Cervical cancer, version 2.2015. J. Natl. Compr. Canc. Netw. 13:395–404; quiz. - PubMed
-
- Minig, L. , Colombo N., Zanagnolo V., Landoni F., Bocciolone L., Cardenas‐Rebollo J. M., et al. 2013. Platinum‐based neoadjuvant chemotherapy followed by radical surgery for cervical carcinoma international federation of gynecology and obstetrics stage IB2‐IIB. Int. J. Gynecol. Cancer 23:1647–1654. - PubMed
-
- Bermudez, A. , Bhatla N., and Leung E.. 2015. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 131(Suppl. 2):S88–S95. - PubMed
-
- Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta‐analysis Collaboration . 2003. Neoadjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta‐analysis of individual patient data from 21 randomised trials. Eur. J. Cancer 39:2470–2486. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

