Dose-escalation study of tabalumab with bortezomib and dexamethasone in Japanese patients with multiple myeloma
- PMID: 27350068
- PMCID: PMC5021044
- DOI: 10.1111/cas.13000
Dose-escalation study of tabalumab with bortezomib and dexamethasone in Japanese patients with multiple myeloma
Abstract
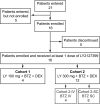
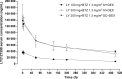
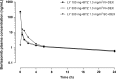
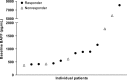
B-cell activating factor (BAFF) promotes the survival and adhesion of multiple myeloma (MM) cells. Tabalumab (LY2127399) is an anti-BAFF monoclonal antibody. This phase 1, multicenter, open-label, nonrandomized, dose-escalation study evaluated the safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of tabalumab in combination with bortezomib and dexamethasone in Japanese patients with relapsed or refractory MM (RRMM). Sixteen patients received intravenous i.v. tabalumab 100 mg (Cohort 1, n = 4) or i.v. tabalumab 300 mg (Cohort 2, n = 12) in combination with oral dexamethasone 20 mg/day and i.v. or s.c. bortezomib 1.3 mg/m(2) . All patients had treatment-emergent adverse events (TEAE) possibly related to study treatment; the most common TEAE were thrombocytopenia (81.3%), lymphopenia (43.8%) and increased alanine aminotransferase (43.8%). Two (20.0%) dose-limiting toxicities were observed, both in Cohort 2 (tabalumab 300 mg), which was below the predefined cutoff for tolerability (<33%). The pharmacokinetics of tabalumab were similar when bortezomib was coadministered i.v. versus s.c. The overall response rate was 56.3%, suggesting that the combined treatment was effective. In conclusion, combined treatment with these three agents was well tolerated in this population of Japanese patients with RRMM. The study was registered at www.clinicaltrials.gov (NCT01556438).
Keywords: Anti-B-cell activating factor monoclonal antibody; LY2127399; multiple myeloma; phase 1 study; tabalumab.
© 2016 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

Similar articles
-
Phase 2 study of tabalumab, a human anti-B-cell activating factor antibody, with bortezomib and dexamethasone in patients with previously treated multiple myeloma.Br J Haematol. 2017 Mar;176(5):783-795. doi: 10.1111/bjh.14483. Epub 2016 Dec 22. Br J Haematol. 2017. PMID: 28005265 Clinical Trial.
-
Safety and efficacy of daratumumab in combination with bortezomib and dexamethasone in Japanese patients with relapsed or refractory multiple myeloma.Int J Hematol. 2018 Apr;107(4):460-467. doi: 10.1007/s12185-017-2390-2. Epub 2017 Dec 19. Int J Hematol. 2018. PMID: 29260507 Clinical Trial.
-
Phase 1 Study of Tabalumab, a Human Anti-B-Cell Activating Factor Antibody, and Bortezomib in Patients with Relapsed/Refractory Multiple Myeloma.Clin Cancer Res. 2016 Dec 1;22(23):5688-5695. doi: 10.1158/1078-0432.CCR-16-0201. Epub 2016 Jun 10. Clin Cancer Res. 2016. PMID: 27287072 Clinical Trial.
-
Retrospective Review of the Use of High-Dose Cyclophosphamide, Bortezomib, Doxorubicin, and Dexamethasone for the Treatment of Multiple Myeloma and Plasma Cell Leukemia.Clin Lymphoma Myeloma Leuk. 2019 Sep;19(9):560-569. doi: 10.1016/j.clml.2019.05.001. Epub 2019 May 17. Clin Lymphoma Myeloma Leuk. 2019. PMID: 31201134 Free PMC article. Review.
-
Immunotherapy: A New Approach to Treating Multiple Myeloma with Daratumumab and Elotuzumab.Ann Pharmacother. 2016 Jul;50(7):555-68. doi: 10.1177/1060028016642786. Epub 2016 Apr 15. Ann Pharmacother. 2016. PMID: 27083916 Free PMC article. Review.
Cited by
-
Anti-B-Cell-Activating Factor (BAFF) Therapy: A Novel Addition to Autoimmune Disease Management and Potential for Immunomodulatory Therapy in Warm Autoimmune Hemolytic Anemia.Biomedicines. 2024 Jul 18;12(7):1597. doi: 10.3390/biomedicines12071597. Biomedicines. 2024. PMID: 39062171 Free PMC article. Review.
-
Current status of BAFF targeting immunotherapy in B-cell neoplasm.Int J Clin Oncol. 2024 Nov;29(11):1676-1683. doi: 10.1007/s10147-024-02611-2. Epub 2024 Sep 2. Int J Clin Oncol. 2024. PMID: 39222149 Free PMC article. Review.
-
Pathogenesis and Treatment of Myeloma-Related Bone Disease.Int J Mol Sci. 2022 Mar 14;23(6):3112. doi: 10.3390/ijms23063112. Int J Mol Sci. 2022. PMID: 35328533 Free PMC article. Review.
-
Investigational Monoclonal Antibodies in the Treatment of Multiple Myeloma: A Systematic Review of Agents under Clinical Development.Antibodies (Basel). 2019 May 24;8(2):34. doi: 10.3390/antib8020034. Antibodies (Basel). 2019. PMID: 31544840 Free PMC article. Review.
-
Mechanisms of Action of the New Antibodies in Use in Multiple Myeloma.Front Oncol. 2021 Jul 8;11:684561. doi: 10.3389/fonc.2021.684561. eCollection 2021. Front Oncol. 2021. PMID: 34307150 Free PMC article. Review.
References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364: 1046–60. - PubMed
-
- Matsuda T, Marugame T, Kamo K, Katanoda K, Ajiki W, Sobue T. Cancer incidence and incidence rates in Japan in 2005: based on data from 12 population‐based cancer registries in the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2011; 41: 139–47. - PubMed
-
- Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol 2009; 9: 491–502. - PubMed
-
- He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, Cerutti A. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol 2004; 172: 3268–79. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

