Functional map of arrestin binding to phosphorylated opsin, with and without agonist
- PMID: 27350090
- PMCID: PMC4923902
- DOI: 10.1038/srep28686
Functional map of arrestin binding to phosphorylated opsin, with and without agonist
Erratum in
-
Corrigendum: Functional map of arrestin binding to phosphorylated opsin, with and without agonist.Sci Rep. 2016 Jul 22;6:30224. doi: 10.1038/srep30224. Sci Rep. 2016. PMID: 27443887 Free PMC article. No abstract available.
Abstract
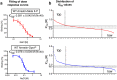
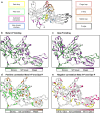
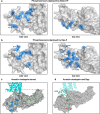
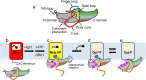
Arrestins desensitize G protein-coupled receptors (GPCRs) and act as mediators of signalling. Here we investigated the interactions of arrestin-1 with two functionally distinct forms of the dim-light photoreceptor rhodopsin. Using unbiased scanning mutagenesis we probed the individual contribution of each arrestin residue to the interaction with the phosphorylated apo-receptor (Ops-P) and the agonist-bound form (Meta II-P). Disruption of the polar core or displacement of the C-tail strengthened binding to both receptor forms. In contrast, mutations of phosphate-binding residues (phosphosensors) suggest the phosphorylated receptor C-terminus binds arrestin differently for Meta II-P and Ops-P. Likewise, mutations within the inter-domain interface, variations in the receptor-binding loops and the C-edge of arrestin reveal different binding modes. In summary, our results indicate that arrestin-1 binding to Meta II-P and Ops-P is similarly dependent on arrestin activation, although the complexes formed with these two receptor forms are structurally distinct.
Figures



Similar articles
-
Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin.Proc Natl Acad Sci U S A. 2013 Jan 15;110(3):942-7. doi: 10.1073/pnas.1215176110. Epub 2012 Dec 31. Proc Natl Acad Sci U S A. 2013. PMID: 23277586 Free PMC article.
-
Quantification of arrestin-rhodopsin binding stoichiometry.Methods Mol Biol. 2015;1271:235-50. doi: 10.1007/978-1-4939-2330-4_16. Methods Mol Biol. 2015. PMID: 25697528
-
Constitutively active rhodopsin mutants causing night blindness are effectively phosphorylated by GRKs but differ in arrestin-1 binding.Cell Signal. 2013 Nov;25(11):2155-62. doi: 10.1016/j.cellsig.2013.07.009. Epub 2013 Jul 17. Cell Signal. 2013. PMID: 23872075 Free PMC article.
-
Structure and self-association of Arrestin-1.J Struct Biol. 2025 Mar;217(1):108173. doi: 10.1016/j.jsb.2025.108173. Epub 2025 Jan 27. J Struct Biol. 2025. PMID: 39880147 Review.
-
The structural basis of the arrestin binding to GPCRs.Mol Cell Endocrinol. 2019 Mar 15;484:34-41. doi: 10.1016/j.mce.2019.01.019. Epub 2019 Jan 28. Mol Cell Endocrinol. 2019. PMID: 30703488 Free PMC article. Review.
Cited by
-
Arrestins: Introducing Signaling Bias Into Multifunctional Proteins.Prog Mol Biol Transl Sci. 2018;160:47-61. doi: 10.1016/bs.pmbts.2018.07.007. Epub 2018 Sep 6. Prog Mol Biol Transl Sci. 2018. PMID: 30470292 Free PMC article. Review.
-
Molecular basis of β-arrestin coupling to formoterol-bound β1-adrenoceptor.Nature. 2020 Jul;583(7818):862-866. doi: 10.1038/s41586-020-2419-1. Epub 2020 Jun 17. Nature. 2020. PMID: 32555462 Free PMC article.
-
Structural Basis of Arrestin Selectivity for Active Phosphorylated G Protein-Coupled Receptors.Int J Mol Sci. 2021 Nov 19;22(22):12481. doi: 10.3390/ijms222212481. Int J Mol Sci. 2021. PMID: 34830362 Free PMC article. Review.
-
How GPCR Phosphorylation Patterns Orchestrate Arrestin-Mediated Signaling.Cell. 2020 Dec 23;183(7):1813-1825.e18. doi: 10.1016/j.cell.2020.11.014. Epub 2020 Dec 8. Cell. 2020. PMID: 33296703 Free PMC article.
-
The Role of Individual Residues in the N-Terminus of Arrestin-1 in Rhodopsin Binding.Int J Mol Sci. 2025 Jan 16;26(2):715. doi: 10.3390/ijms26020715. Int J Mol Sci. 2025. PMID: 39859432 Free PMC article.
References
-
- Fredriksson R., Lagerström M. C., Lundin L.-G. & Schiöth H. B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272 (2003). - PubMed
-
- Lefkowitz R. J. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 190, 9–19 (2007). - PubMed
-
- Schöneberg T. T. et al.. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol. Ther. 104, 173–206 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

