Prolonged Culture of Aligned Skeletal Myotubes on Micromolded Gelatin Hydrogels
- PMID: 27350122
- PMCID: PMC4924097
- DOI: 10.1038/srep28855
Prolonged Culture of Aligned Skeletal Myotubes on Micromolded Gelatin Hydrogels
Abstract
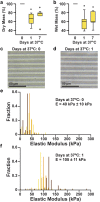
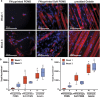
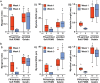
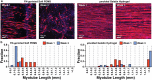
In vitro models of skeletal muscle are critically needed to elucidate disease mechanisms, identify therapeutic targets, and test drugs pre-clinically. However, culturing skeletal muscle has been challenging due to myotube delamination from synthetic culture substrates approximately one week after initiating differentiation from myoblasts. In this study, we successfully maintained aligned skeletal myotubes differentiated from C2C12 mouse skeletal myoblasts for three weeks by utilizing micromolded (μmolded) gelatin hydrogels as culture substrates, which we thoroughly characterized using atomic force microscopy (AFM). Compared to polydimethylsiloxane (PDMS) microcontact printed (μprinted) with fibronectin (FN), cell adhesion on gelatin hydrogel constructs was significantly higher one week and three weeks after initiating differentiation. Delamination from FN-μprinted PDMS precluded robust detection of myotubes. Compared to a softer blend of PDMS μprinted with FN, myogenic index, myotube width, and myotube length on μmolded gelatin hydrogels was similar one week after initiating differentiation. However, three weeks after initiating differentiation, these parameters were significantly higher on μmolded gelatin hydrogels compared to FN-μprinted soft PDMS constructs. Similar results were observed on isotropic versions of each substrate, suggesting that these findings are independent of substrate patterning. Our platform enables novel studies into skeletal muscle development and disease and chronic drug testing in vitro.
Figures



References
-
- Wang Z. et al. Muscularity in adult humans: proportion of adipose tissue-free body mass as skeletal muscle. American journal of human biology: the official journal of the Human Biology Council. 13, 612–619 (2001). - PubMed
-
- Spurney C. F. Cardiomyopathy of Duchenne muscular dystrophy: current understanding and future directions. Muscle & nerve. 44, 8–19 (2011). - PubMed
-
- Cholewa J. et al. Basic models modeling resistance training: an update for basic scientists interested in study skeletal muscle hypertrophy. Journal of cellular physiology. 229, 1148–1156 (2014). - PubMed
-
- Rai M., Nongthomba U. & Grounds M. D. Skeletal muscle degeneration and regeneration in mice and flies. Current topics in developmental biology. 108, 247–281 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

