Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) and serine biosynthetic pathway genes are co-ordinately increased during anabolic agent-induced skeletal muscle growth
- PMID: 27350173
- PMCID: PMC4923900
- DOI: 10.1038/srep28693
Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) and serine biosynthetic pathway genes are co-ordinately increased during anabolic agent-induced skeletal muscle growth
Abstract
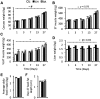
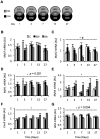
We aimed to identify novel molecular mechanisms for muscle growth during administration of anabolic agents. Growing pigs (Duroc/(Landrace/Large-White)) were administered Ractopamine (a beta-adrenergic agonist; BA; 20 ppm in feed) or Reporcin (recombinant growth hormone; GH; 10 mg/48 hours injected) and compared to a control cohort (feed only; no injections) over a 27-day time course (1, 3, 7, 13 or 27-days). Longissimus Dorsi muscle gene expression was analyzed using Agilent porcine transcriptome microarrays and clusters of genes displaying similar expression profiles were identified using a modified maSigPro clustering algorithm. Anabolic agents increased carcass (p = 0.002) and muscle weights (Vastus Lateralis: p < 0.001; Semitendinosus: p = 0.075). Skeletal muscle mRNA expression of serine/one-carbon/glycine biosynthesis pathway genes (Phgdh, Psat1 and Psph) and the gluconeogenic enzyme, phosphoenolpyruvate carboxykinase-M (Pck2/PEPCK-M), increased during treatment with BA, and to a lesser extent GH (p < 0.001, treatment x time interaction). Treatment with BA, but not GH, caused a 2-fold increase in phosphoglycerate dehydrogenase (PHGDH) protein expression at days 3 (p < 0.05) and 7 (p < 0.01), and a 2-fold increase in PEPCK-M protein expression at day 7 (p < 0.01). BA treated pigs exhibit a profound increase in expression of PHGDH and PEPCK-M in skeletal muscle, implicating a role for biosynthetic metabolic pathways in muscle growth.
Figures




References
-
- Sandri M. Signaling in Muscle Atrophy and Hypertrophy. Physiology 23, 160–170 (2008). - PubMed
-
- Buttery P. J. & Dawson J. M. Growth promotion in farm animals. Proc. Nutr. Soc. 49, 459–66 (1990). - PubMed
-
- Lynch G. S. & Ryall J. G. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol. Rev. 88, 729–767 (2008). - PubMed
-
- Dunshea F. R., King R. H., Campbell R. G., Sainz R. D. & Kim Y. S. Interrelationships between sex and ractopamine on protein and lipid deposition in rapidly growing pigs. J. Anim. Sci. 71, 2919–30 (1993). - PubMed
-
- Boisclair Y. R., Bauman D. E., Bell A. W., Dunshea F. R. & Harkins M. Nutrient utilization and protein turnover in the hindlimb of cattle treated with bovine somatotropin. J Nutr 124, 664–673 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

