Angiopellosis as an Alternative Mechanism of Cell Extravasation
- PMID: 27350343
- PMCID: PMC5376103
- DOI: 10.1002/stem.2451
Angiopellosis as an Alternative Mechanism of Cell Extravasation
Abstract
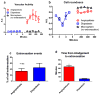
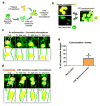
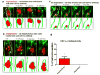
Stem cells possess the ability to home in and travel to damaged tissue when injected intravenously. For the cells to exert their therapeutic effect, they must cross the blood vessel wall and enter the surrounding tissues. The mechanism of extravasation injected stem cells employ for exit has yet to be characterized. Using intravital microscopy and a transgenic zebrafish line Tg(fli1a:egpf) with GFP-expressing vasculature, we documented the detailed extravasation processes in vivo for injected stem cells in comparison to white blood cells (WBCs). While WBCs left the blood vessels by the standard diapedesis process, injected cardiac and mesenchymal stem cells underwent a distinct method of extravasation that was markedly different from diapedesis. Here, the vascular wall undergoes an extensive remodeling to allow the cell to exit the lumen, while the injected cell remains distinctively passive in activity. We termed this process Angio-pello-sis, which represents an alternative mechanism of cell extravasation to the prevailing theory of diapedesis. Stem Cells 2017;35:170-180 Video Highlight: https://youtu.be/i5EI-ZvhBps.
Keywords: Angiopellosis; Diapedesis; Extravasation; Stem cell infusion; Transmigration.
© 2016 AlphaMed Press.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

