New ursane triterpenoids from Ficus pandurata and their binding affinity for human cannabinoid and opioid receptors
- PMID: 27350550
- PMCID: PMC5590819
- DOI: 10.1007/s12272-016-0784-y
New ursane triterpenoids from Ficus pandurata and their binding affinity for human cannabinoid and opioid receptors
Abstract
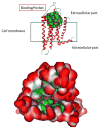
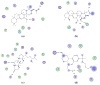
Phytochemical investigation of Ficus pandurata Hance (Moraceae) fruits has led to the isolation of two new triterpenoids, ficupanduratin A [1β-hydroxy-3β-acetoxy-11α-methoxy-urs-12-ene] (11) and ficupanduratin B [21α-hydroxy-3β-acetoxy-11α-methoxy-urs-12-ene] (17), along with 20 known compounds: α-amyrin acetate (1), α-amyrin (2), 3β-acetoxy-20-taraxasten-22-one (3), 3β-acetoxy-11α-methoxy-olean-12-ene (4), 3β-acetoxy-11α-methoxy-12-ursene (5), 11-oxo-α-amyrin acetate (6), 11-oxo-β-amyrin acetate (7), palmitic acid (8), stigmast-4,22-diene-3,6-dione (9), stigmast-4-ene-3,6-dione (10), stigmasterol (12), β-sitosterol (13), stigmast-22-ene-3,6-dione (14), stigmastane-3,6-dione (15), 3β,21β-dihydroxy-11α-methoxy-olean-12-ene (16), 3β-hydroxy-11α-methoxyurs-12-ene (18), 6-hydroxystigmast-4,22-diene-3-one (19), 6-hydroxystigmast-4-ene-3-one (20), 11α,21α-dihydroxy-3β-acetoxy-urs-12-ene (21), and β-sitosterol-3-O-β-D-glucopyranoside (22). Compound 21 is reported for the first time from a natural source. The structures of the 20 compounds were elucidated on the basis of IR, 1D ((1)H and (13)C), 2D ((1)H-(1)H COSY, HSQC, HMBC and NOESY) NMR and MS spectroscopic data, in addition to comparison with literature data. The isolated compounds were evaluated for their anti-microbial, anti-malarial, anti-leishmanial, and cytotoxic activities. In addition, their radioligand displacement affinity on opioid and cannabinoid receptors was assessed. Compounds 4, 11, and 15 exhibited good affinity towards the CB2 receptor, with displacement values of 69.7, 62.5 and 86.5 %, respectively. Furthermore, the binding mode of the active compounds in the active site of the CB2 cannabinoid receptors was investigated through molecular modelling.
Keywords: Anti-leishmanial; Anti-malarial; Cannabinoid receptors; Ficus pandurata; Opioid receptors; Triterpenes.
Conflict of interest statement
Figures



References
-
- Abood ME. Molecular biology of cannabinoid receptors. Handbook Exp Pharmacol. 2005;168:81–115. - PubMed
-
- Ahmed AS. Pentacyclic triterpenes from Ficus pandurata Hance fruit. Bull Pharm Sci Assiut Univ. 2010;33:1–7.
-
- Ajoku GA, Okwute SK, Okogun JI. Isolation of hexadecanoic acid methyl ester and 1,1,2-ethanetricarboxylic acid-1-hydroxy-1, 1-dimethyl ester from the calyx of Green Hibiscus Sabdariffa (Linn) Nat Prod Chem Res. 2015;3:1–5.
-
- Al Musayeib NM, Mothana RA, Ibrahim SRM, El Gamal AA, Al-Massarani SM. Klodorone A and klodorol A: new triterpenes from Kleinia odora. Nat Prod Res. 2014;28:1142–1146. - PubMed
-
- Al-Attas AAM, El-Shaer NS, Mohamed GA, Ibrahim SRM, Esmat A. Anti-inflammatory sesquiterpenes from Costus speciosus rhizomes. J Ethnopharmacol. 2015;176:365–374. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

