Transient receptor potential vanilloid 4-dependent calcium influx and ATP release in mouse and rat gastric epithelia
- PMID: 27350729
- PMCID: PMC4917611
- DOI: 10.3748/wjg.v22.i24.5512
Transient receptor potential vanilloid 4-dependent calcium influx and ATP release in mouse and rat gastric epithelia
Abstract
Aim: To explore the expression of transient receptor potential vanilloid 4 (TRPV4) and its physiological meaning in mouse and rat gastric epithelia.
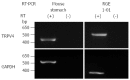
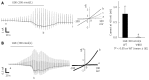
Methods: RT-PCR and immunochemistry were used to detect TRPV4 mRNA and protein expression in mouse stomach and a rat normal gastric epithelial cell line (RGE1-01), while Ca(2+)-imaging and electrophysiology were used to evaluate TRPV4 channel activity. ATP release was measured by a luciferin-luciferase assay. Gastric emptying was also compared between WT and TRPV4 knockout mice.
Results: TRPV4 mRNA and protein were detected in mouse tissues and RGE1-01 cells. A TRPV4-specific agonist (GSK1016790A) increased intracellular Ca(2+) concentrations and/or evoked TRPV4-like current activities in WT mouse gastric epithelial cells and RGE1-01 cells, but not TRPV4KO cells. GSK1016790A or mechanical stimuli induced ATP release from RGE1-01 cells while TRPV4 knockout mice displayed delayed gastric emptying in vivo.
Conclusion: TRPV4 is expressed in mouse and rat gastric epithelium and contributes to ATP release and gastric emptying.
Keywords: ATP; Gastric emptying; Stomach; Transient receptor potential vanilloid 4.
Figures




References
-
- Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol. 2010;103:2–17. - PubMed
-
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. - PubMed
-
- Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, et al. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther. 2008;326:443–452. - PubMed
-
- D’Aldebert E, Cenac N, Rousset P, Martin L, Rolland C, Chapman K, Selves J, Alric L, Vinel JP, Vergnolle N. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011;140:275–285. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

