Intravoxel incoherent motion diffusion-weighted imaging for monitoring chemotherapeutic efficacy in gastric cancer
- PMID: 27350730
- PMCID: PMC4917612
- DOI: 10.3748/wjg.v22.i24.5520
Intravoxel incoherent motion diffusion-weighted imaging for monitoring chemotherapeutic efficacy in gastric cancer
Abstract
Aim: To assess intravoxel incoherent motion diffusion-weighted imaging (IVIM-DWI) for monitoring early efficacy of chemotherapy in a human gastric cancer mouse model.
Methods: IVIM-DWI was performed with 12 b-values (0-800 s/mm(2)) in 25 human gastric cancer-bearing nude mice at baseline (day 0), and then they were randomly divided into control and 1-, 3-, 5- and 7-d treatment groups (n = 5 per group). The control group underwent longitudinal MRI scans at days 1, 3, 5 and 7, and the treatment groups underwent subsequent MRI scans after a specified 5-fluorouracil/calcium folinate treatment. Together with tumor volumes (TV), the apparent diffusion coefficient (ADC) and IVIM parameters [true water molecular diffusion coefficient (D), perfusion fraction (f) and pseudo-related diffusion coefficient (D(*))] were measured. The differences in those parameters from baseline to each measurement (ΔTV%, ΔADC%, ΔD%, Δf% and ΔD(*)%) were calculated. After image acquisition, tumor necrosis, microvessel density (MVD) and cellular apoptosis were evaluated by hematoxylin-eosin (HE), CD31 and terminal-deoxynucleotidyl transferase mediated nick end labeling (TUNEL) staining respectively, to confirm the imaging findings. Mann-Whitney test and Spearman's correlation coefficient analysis were performed.
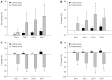
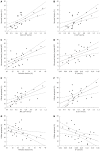
Results: The observed relative volume increase (ΔTV%) in the treatment group were significantly smaller than those in the control group at day 5 (ΔTVtreatment% = 19.63% ± 3.01% and ΔTVcontrol% = 83.60% ± 14.87%, P = 0.008) and day 7 (ΔTVtreatment% = 29.07% ± 10.01% and ΔTVcontrol% = 177.06% ± 63.00%, P = 0.008). The difference in ΔTV% between the treatment and the control groups was not significant at days 1 and 3 after a short duration of treatment. Increases in ADC in the treatment group (ΔADC%treatment, median, 30.10% ± 18.32%, 36.11% ± 21.82%, 45.22% ± 24.36%) were significantly higher compared with the control group (ΔADC%control, median, 4.98% ± 3.39%, 6.26% ± 3.08%, 9.24% ± 6.33%) at days 3, 5 and 7 (P = 0.008, P = 0.016, P = 0.008, respectively). Increases in D in the treatment group (ΔD%treatment, median 17.12% ± 8.20%, 24.16% ± 16.87%, 38.54% ± 19.36%) were higher than those in the control group (ΔD%control, median -0.13% ± 4.23%, 5.89% ± 4.56%, 5.54% ± 4.44%) at days 1, 3, and 5 (P = 0.032, P = 0.008, P = 0.016, respectively). Relative changes in f were significantly lower in the treatment group compared with the control group at days 1, 3, 5 and 7 follow-up (median, -34.13% ± 16.61% vs 1.68% ± 3.40%, P = 0.016; -50.64% ± 6.82% vs 3.01% ± 6.50%, P = 0.008; -49.93% ± 6.05% vs 0.97% ± 4.38%, P = 0.008, and -46.22% ± 7.75% vs 8.14% ± 6.75%, P = 0.008, respectively). D* in the treatment group decreased significantly compared to those in the control group at all time points (median, -32.10% ± 12.22% vs 1.85% ± 5.54%, P = 0.008; -44.14% ± 14.83% vs 2.29% ± 10.38%, P = 0.008; -59.06% ± 19.10% vs 3.86% ± 5.10%, P = 0.008 and -47.20% ± 20.48% vs 7.13% ± 9.88%, P = 0.016, respectively). Furthermore, histopathologic findings showed positive correlations with ADC and D and tumor necrosis (r s = 0.720, P < 0.001; r s = 0.522, P = 0.007, respectively). The cellular apoptosis of the tumor also showed positive correlations with ADC and D (r s = 0.626, P = 0.001; r s = 0.542, P = 0.005, respectively). Perfusion-related parameters (f and D(*)) were positively correlated to MVD (r s = 0.618, P = 0.001; r s = 0.538, P = 0.006, respectively), and negatively correlated to cellular apoptosis of the tumor (r s = -0.550, P = 0.004; r s = -0.692, P < 0.001, respectively).
Conclusion: IVIM-DWI is potentially useful for predicting the early efficacy of chemotherapy in a human gastric cancer mouse model.
Keywords: Gastric cancer; Intravoxel incoherent motion diffusion-weighted imaging; Microvessel density; Nude mouse model; Terminal-deoxynucleoitidyl transferase mediated nick end labeling.
Figures



Similar articles
-
Chemotherapy response evaluation in a mouse model of gastric cancer using intravoxel incoherent motion diffusion-weighted MRI and histopathology.World J Gastroenterol. 2017 Mar 21;23(11):1990-2001. doi: 10.3748/wjg.v23.i11.1990. World J Gastroenterol. 2017. PMID: 28373765 Free PMC article.
-
[Evaluation of tumor vascular normalization in colorectal cancer mouse mode induced by recombinant human endostatin by intravoxel incoherent motion diffusion-weighted magnetic resonance imaging].Zhonghua Zhong Liu Za Zhi. 2019 Jun 23;41(6):421-428. doi: 10.3760/cma.j.issn.0253-3766.2019.06.005. Zhonghua Zhong Liu Za Zhi. 2019. PMID: 31216827 Chinese.
-
Quantitative intravoxel incoherent motion parameters derived from whole-tumor volume for assessing pathological complete response to neoadjuvant chemotherapy in locally advanced rectal cancer.J Magn Reson Imaging. 2018 Jul;48(1):248-258. doi: 10.1002/jmri.25931. Epub 2017 Dec 27. J Magn Reson Imaging. 2018. PMID: 29281151
-
Intravoxel Incoherent Motion Diffusion-Weighted Imaging Used to Detect Prostate Cancer and Stratify Tumor Grade: A Meta-Analysis.Front Oncol. 2020 Sep 11;10:1623. doi: 10.3389/fonc.2020.01623. eCollection 2020. Front Oncol. 2020. PMID: 33042805 Free PMC article.
-
[Diffusion-weighted imaging of the pancreas].Radiologe. 2011 Mar;51(3):186-94. doi: 10.1007/s00117-010-2059-9. Radiologe. 2011. PMID: 21305263 Review. German.
Cited by
-
Correlation between IVIM parameters and microvessel architecture: direct comparison of MRI images and pathological slices in an orthotopic murine model of rhabdomyosarcoma.Eur Radiol. 2023 Dec;33(12):8576-8584. doi: 10.1007/s00330-023-09835-2. Epub 2023 Jun 27. Eur Radiol. 2023. PMID: 37368112
-
Application of intravoxel incoherent motion diffusion-weighted imaging for preoperative knowledge of lymphovascular invasion in gastric cancer: a prospective study.Abdom Radiol (NY). 2023 Jul;48(7):2207-2218. doi: 10.1007/s00261-023-03920-2. Epub 2023 Apr 21. Abdom Radiol (NY). 2023. PMID: 37085731
-
Predicting tumor invasion depth in gastric cancer: developing and validating multivariate models incorporating preoperative IVIM-DWI parameters and MRI morphological characteristics.Eur J Med Res. 2024 Aug 22;29(1):431. doi: 10.1186/s40001-024-02017-w. Eur J Med Res. 2024. PMID: 39175075 Free PMC article.
-
IVIM improves preoperative assessment of microvascular invasion in HCC.Eur Radiol. 2019 Oct;29(10):5403-5414. doi: 10.1007/s00330-019-06088-w. Epub 2019 Mar 15. Eur Radiol. 2019. PMID: 30877465
-
Intravoxel incoherent motion imaging used to assess tumor microvascular changes after transarterial chemoembolization in a rabbit VX2 liver tumor model.Front Oncol. 2023 Feb 28;13:1114406. doi: 10.3389/fonc.2023.1114406. eCollection 2023. Front Oncol. 2023. PMID: 36925931 Free PMC article.
References
-
- Spampatti S, Rausei S, Galli F, Ruspi L, Peverelli C, Frattini F, Rovera F, Boni L, Dionigi G. Neoadjuvant chemotherapy for locally advanced gastric cancer: the surgeon’s role. Transl Gastrointest Cancer. 2015;4:141–147.
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

