Antinociceptive effects of topical mepivacaine in a rat model of HIV-associated peripheral neuropathic pain
- PMID: 27350758
- PMCID: PMC4902250
- DOI: 10.2147/JPR.S104397
Antinociceptive effects of topical mepivacaine in a rat model of HIV-associated peripheral neuropathic pain
Abstract
Background: A consequence of HIV infection is sensory neuropathy, a debilitating condition that degrades the quality of life of HIV patients. Furthermore, life-extending antiretroviral treatment may exacerbate HIV sensory neuropathy. Analgesics that relieve other neuropathic pains show little or no efficacy in ameliorating HIV sensory neuropathy. Thus, there is a need for analgesics for people with this particular pain. While lidocaine is used in the management of painful peripheral neuropathies, another local anesthetic mepivacaine, with a potentially improved bioavailability, could be utilized for the management of HIV neuropathic pain.
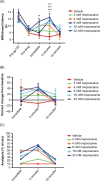
Methods: The efficacy of topical anesthetics was evaluated in a preclinical rodent model of painful peripheral neuropathy induced by epineural administration of the HIV envelope protein gp120 delivered using saturated oxidized cellulose implanted around the sciatic nerve. Beginning at 2 weeks following gp120 administration, the effects of local anesthetics topically applied via gauze pads were tested on heat and mechanical hyperalgesia in the hind paw. Rats were tested using several concentrations of mepivacaine or lidocaine during the following 2 weeks.
Results: By 2 weeks following epineural gp120 implantation, the ipsilateral hind paw developed significant hypersensitivity to noxious pressure and heat hyperalgesia. A short-lasting, concentration-dependent amelioration of pressure and heat hyperalgesia was observed following topical application of mepivacaine to the ipsilateral plantar hind paw. By contrast, topical lidocaine ameliorated heat hyperalgesia in a concentration-dependent manner but not pressure hyperalgesia. Equipotent concentrations of mepivacaine and lidocaine applied topically to the tail of mice significantly increased tail withdrawal latencies in the tail flick test, demonstrating that both local anesthetics attenuate responding to a brief noxious stimulus.
Conclusion: These findings showed that mepivacaine, rather than lidocaine, consistently attenuated two distinct symptoms of neuropathic pain and suggest that topical formulations of this local anesthetic could have utility in the alleviation of clinical HIV neuropathic pain.
Keywords: AIDs-related pain; acute pain; analgesia; chronic pain; distal sensory neuropathy; local anesthetics.
Figures




References
-
- Freeman R, Baron R, Bouhassiara D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on neuropathic pain symptoms and signs. Pain. 2014;155:367–376. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

