Modified Ames test using a strain expressing human sulfotransferase 1C2 to assess the mutagenicity of methyleugenol
- PMID: 27350821
- PMCID: PMC4918123
- DOI: 10.1186/s41021-016-0028-x
Modified Ames test using a strain expressing human sulfotransferase 1C2 to assess the mutagenicity of methyleugenol
Abstract
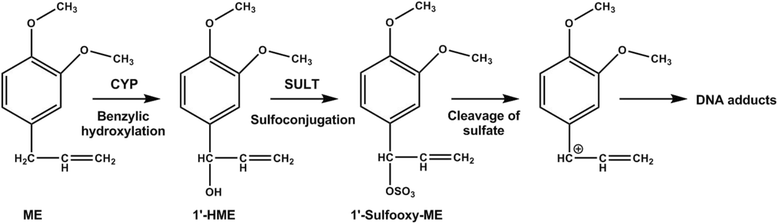
Introduction: Several alkenylbenzenes, including methyleugenol (ME), are present in a wide range of botanicals and exhibit carcinogenic and mutagenic properties. Negative results are generally obtained for alkenylbenzenes in standard in vitro genotoxicity tests, including the Ames test. A lack of mutagenicity observed in such tests is thought to result from impaired metabolic activation of alkenylbenzenes via hydroxylation, with subsequent sulfoconjugation to its ultimate mutagenic or carcinogenic form. Although recent studies have reported the mutagenicity of hydroxylated ME metabolites in the Ames test using modified TA100 strains expressing human sulfotransferases (SULTs), to our knowledge, the detection of ME mutagenicity has not yet been reported.
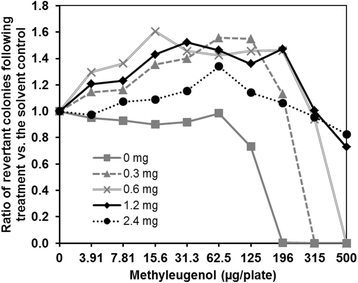
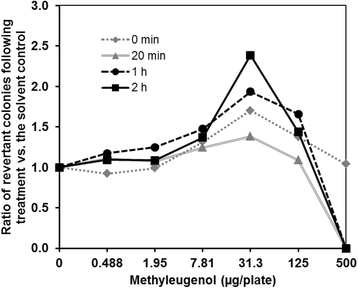
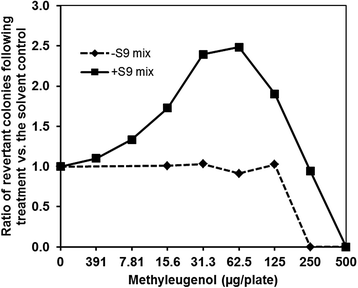
Findings: Using strain TA100-hSULT1C2, which expresses human SULT1C2, we optimized the protein content of S9 Mix and the pre-incubation time required to promote metabolic activation in the Ames test. This procedure enabled us to obtain a positive response with ME.
Conclusions: We established Ames-test conditions enabling the detection of ME-induced mutagenicity, using a strain expressing human SULT1C2 in the presence of induced-rat S9 Mix. This simple approach will help assess the mutagenicity of other alkenylbenzenes and related chemicals.
Keywords: Alkenylbenzene; Ames test; Methyleugenol; S9; Sulfotransferase.
Figures
References
-
- van den Berg SJPL, Restani P, Boersma MG, Delmulle L, Rietjens IMCM. Levels of genotoxic and carcinogenic ingredients in plant food supplements and associated risk assessment. Food Nutr Sci. 2011;2:989–1010. doi: 10.4236/fns.2011.29134. - DOI
-
- National Toxicology Program NTP toxicology and carcinogenesis studies of methyleugenol (CAS NO. 93-15-2) in F344/N rats and B6C3F1 mice (gavage studies) Natl Toxicol Progr Tech Rep Ser. 2000;491:1–412. - PubMed
-
- World Health Organization. IARC monographs on the evaluation of carcinogenic risk of chemicals to man volume 1. Natural products: safrole, isosafrole and dihydrosafrole. 1972. http://monographs.iarc.fr/ENG/Monographs/vol1-42/mono1.pdf. Accessed July 24, 2015.
-
- Miller EC, Swanson AB, Phillips DH, Fletcher TL, Liem A, Miller JA. Structure-activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 1983;43:1124–34. - PubMed
-
- IARC. IARC monographs on the evaluation of carcinogenic risks to humans volume 101. Some chemicals present in industrial and consumer products, food and drinking-water. Methyleugenol. 2012. http://monographs.iarc.fr/ENG/Monographs/vol101/mono101-013.pdf Accessed July 24, 2015. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

