Detection of dengue, west Nile virus, rickettsiosis and leptospirosis by a new real-time PCR strategy
- PMID: 27350908
- PMCID: PMC4899400
- DOI: 10.1186/s40064-016-2318-y
Detection of dengue, west Nile virus, rickettsiosis and leptospirosis by a new real-time PCR strategy
Abstract
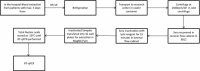
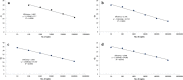
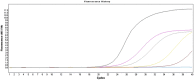
Dengue virus (DENV) infection causes sudden fever along with several nonspecific signs and symptoms and in severe cases, death. DENV is transmitted to people by Aedes aegypti and Ae. albopictus mosquitoes, whose populations increase during rainy season. West Nile Virus (WNV), Rickettsia spp. and Leptospira spp. are fever-causing pathogens that share many of the initial symptoms of DENV infection and also thrive in the rainy season. Outbreaks in some regions may be due to any of these pathogens that can co-circulate. Plus, they are clinically indistinguishable until severe symptoms appear, even though these diseases should be treated differently. An effective differential diagnosis would help clinicians and vector control departments to make right decisions for control and treatment of these diseases. Therefore, we developed four different SYBR green (®) -based reverse transcription quantitative PCR (RT-qPCR) assays for simultaneous detection of DENV, WNV, Rickettsia spp. and Leptospira spp. The assay has been optimized to yield results in less than 1 h; and in order to reduce contamination risk, all reagents were premixed and lyophilized on 96 well plates and thus only requires the addition of water and total nucleic acids from the sample. Sensitivities of the assays were less than 100 copies of nucleic acid targeted for these four pathogens. Assays did not show cross reactivity with any of the four pathogens nor to human nucleic acids. We are presenting a sensitive and selective kit that detects four relevant pathogens from tropical regions, that is quick, cost-effective and easy to use.
Keywords: Dengue; Leptospira; Real time PCR; Rickettsia; West Nile Virus.
Figures

Similar articles
-
Entomo-virological surveillance strategy for dengue, Zika and chikungunya arboviruses in field-caught Aedes mosquitoes in an endemic urban area of the Northeast of Brazil.Acta Trop. 2019 Sep;197:105061. doi: 10.1016/j.actatropica.2019.105061. Epub 2019 Jun 10. Acta Trop. 2019. PMID: 31194961
-
Vertical transmission of dengue virus in Aedes aegypti and its role in the epidemiological persistence of dengue in Central and Southern Mexico.Trop Med Int Health. 2019 Nov;24(11):1311-1319. doi: 10.1111/tmi.13306. Epub 2019 Oct 8. Trop Med Int Health. 2019. PMID: 31483936
-
Laboratory and surveillance studies following a suspected Dengue case in Greece, 2012.Int J Infect Dis. 2015 Jan;30:150-3. doi: 10.1016/j.ijid.2014.11.019. Epub 2014 Nov 26. Int J Infect Dis. 2015. PMID: 25481048
-
Screening of dengue virus in field-caught Aedes aegypti and Aedes albopictus (Diptera: Culicidae) by one-step SYBR green-based reverse transcriptase-polymerase chain reaction assay during 2004-2007 in Southern Taiwan.Vector Borne Zoonotic Dis. 2010 Dec;10(10):1017-25. doi: 10.1089/vbz.2008.0069. Epub 2010 May 18. Vector Borne Zoonotic Dis. 2010. PMID: 21128850
-
West Nile virus: the Indian scenario.Indian J Med Res. 2003 Sep;118:101-8. Indian J Med Res. 2003. PMID: 14700342 Review.
Cited by
-
Molecular Characterization of Associated Pathogens in Febrile Patients during Inter-Epidemic Periods of Urban Arboviral Diseases in Tapachula Southern Mexico.Pathogens. 2021 Nov 8;10(11):1450. doi: 10.3390/pathogens10111450. Pathogens. 2021. PMID: 34832606 Free PMC article.
-
An outbreak of leptospirosis among reserve military recruits, Hulu Perdik, Malaysia.Eur J Clin Microbiol Infect Dis. 2019 Mar;38(3):523-528. doi: 10.1007/s10096-018-03450-6. Epub 2019 Jan 24. Eur J Clin Microbiol Infect Dis. 2019. PMID: 30680558
-
Assessing the risk of West Nile Virus seasonal outbreaks and its vector control in an urbanizing bird community: An integrative R0-modelling study in the city of Merida, Mexico.PLoS Negl Trop Dis. 2023 May 30;17(5):e0011340. doi: 10.1371/journal.pntd.0011340. eCollection 2023 May. PLoS Negl Trop Dis. 2023. PMID: 37253060 Free PMC article.
-
Characterization of Leptospira isolates from humans and the environment in Uruguay.Rev Inst Med Trop Sao Paulo. 2017 Dec 21;59:e79. doi: 10.1590/S1678-9946201759079. Rev Inst Med Trop Sao Paulo. 2017. PMID: 29267587 Free PMC article.
-
Prevalence of plasmodium, leptospira and rickettsia species in Northern Tanzania: a community based survey.Afr Health Sci. 2020 Mar;20(1):199-207. doi: 10.4314/ahs.v20i1.25. Afr Health Sci. 2020. PMID: 33402908 Free PMC article.
References
-
- Boletín Epidemiológico. Semana 52. Secretaría de Salud. (2016) Mexico DF, Mexico
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

