Antiestrogenic and Anti-Inflammatory Potential of n-Hexane Fraction of Vitex negundo Linn Leaf Extract: A Probable Mechanism for Blastocyst Implantation Failure in Mus musculus
- PMID: 27351007
- PMCID: PMC4897515
- DOI: 10.1155/2014/241946
Antiestrogenic and Anti-Inflammatory Potential of n-Hexane Fraction of Vitex negundo Linn Leaf Extract: A Probable Mechanism for Blastocyst Implantation Failure in Mus musculus
Abstract
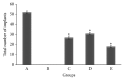
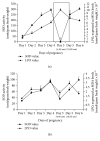
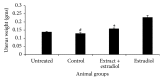
The anti-implantation potential of different fractions of Vitex negundo Linn leaf extract was evaluated in female Swiss Albino mice. Animals from different groups were dosed orally either with 0.2% agar (vehicle) or with fractions of V. negundo leaf extract (n-hexane, chloroform, n-butanol, and remnant fractions) at 10:00 a.m., from day 1 to day 6 of pregnancy. The pregnant females from each group were sacrificed on different days of pregnancy (n = 6), and uterus was excised and used for estimation of lipid peroxidation and assay of superoxide dismutase activity as a marker for blastocyst implantation. Animals treated with n-hexane fraction showed altered level of superoxide anion radical and superoxide dismutase activity as compared to control animals. The probable mechanism by which this extract exhibits inhibition of blastocyst implantation is through the anti-inflammatory and antiestrogenic potential.
Figures

References
-
- Pal A. K., Bhattacharya K., Kabir S. N., Pakrashi A. Flowers of Hibiscus rosa-sinensis, a potential source of contragestative agent: II. possible mode of action with reference to anti-implant effect of the benzene extract. Contraception. 1985;32(5):517–529. doi: 10.1016/0010-7824(85)90021-6. - DOI - PubMed
-
- Psychoyos A. Endocrine control of egg implantation. In: Greep R. O., Astwood E. B., editors. Handbook of Physiology, Section 7, Endocrinology. Vol. 2. Washington, DC, USA: American Physiological Society; 1973. pp. 187–215.
LinkOut - more resources
Full Text Sources
Other Literature Sources

