Inhibition of mast cell-secreted histamine decreases biliary proliferation and fibrosis in primary sclerosing cholangitis Mdr2(-/-) mice
- PMID: 27351144
- PMCID: PMC5033697
- DOI: 10.1002/hep.28704
Inhibition of mast cell-secreted histamine decreases biliary proliferation and fibrosis in primary sclerosing cholangitis Mdr2(-/-) mice
Abstract
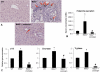
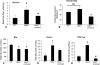
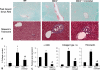
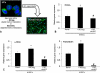
Hepatic fibrosis is marked by activation of hepatic stellate cells (HSCs). Cholestatic injury precedes liver fibrosis, and cholangiocytes interact with HSCs promoting fibrosis. Mast cells (MCs) infiltrate following liver injury and release histamine, increasing biliary proliferation. We evaluated if inhibition of MC-derived histamine decreases biliary proliferation and fibrosis. Wild-type and multidrug resistance 2 knockout mice (9-11 weeks) were treated with cromolyn sodium for 1 week to block MC-derived histamine. Biliary mass and proliferation were evaluated by immunohistochemistry for cytokeratin 19 and Ki-67. Bile flow, bicarbonate excretion, and total bile acids were measured in all mice. Fibrosis was evaluated by sirius red/fast green staining and by quantitative polymerase chain reaction for alpha-smooth muscle actin, fibronectin, collagen type 1a, and transforming growth factor-beta 1. HSC activation was evaluated by quantitative polymerase chain reaction in total liver and immunofluorescent staining in tissues for synaptophysin 9. Histamine serum secretion was measured by enzymatic immunoassay. Mouse liver and human liver samples from control or primary sclerosing cholangitis patients were evaluated for MC markers by quantitative polymerase chain reaction and immunohistochemistry. In vitro, cultured MCs were transfected with histidine decarboxylase short hairpin RNA to decrease histamine secretion and subsequently cocultured with cholangiocytes or HSCs prior to measuring fibrosis markers, proliferation, and transforming growth factor-beta 1 secretion. Treatment with cromolyn sodium decreased biliary proliferation, fibrosis, histamine secretion, and bile flow in multidrug resistance 2 knockout mice. Primary sclerosing cholangitis mice and patients have increased MCs. Knockdown of MC histidine decarboxylase decreased cholangiocyte and HSC proliferation/activation.
Conclusion: MCs are recruited to proliferating cholangiocytes and promote fibrosis. Inhibition of MC-derived histamine decreases fibrosis, and regulation of MC mediators may be therapeutic for primary sclerosing cholangitis. (Hepatology 2016;64:1202-1216).
© 2016 by the American Association for the Study of Liver Diseases. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Figures




References
-
- Angulo P, Peter JB, Gershwin ME, DeSotel CK, Shoenfeld Y, Ahmed AE, Lindor KD. Serum autoantibodies in patients with primary sclerosing cholangitis. J Hepatol. 2000;32:182–187. - PubMed
-
- Osterreicher CH, Trauner M. Animal models of biliary tract injury. Curr Opin Gastroenterol. 2012;28:239–243. - PubMed
-
- Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. - PubMed
-
- Trauner M, Fickert P, Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007;27:77–98. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

