Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer
- PMID: 27351217
- PMCID: PMC4947701
- DOI: 10.1038/bjc.2016.185
Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer
Erratum in
-
Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer.Br J Cancer. 2016 Oct 25;115(9):e13. doi: 10.1038/bjc.2016.306. Epub 2016 Sep 22. Br J Cancer. 2016. PMID: 27657342 Free PMC article. No abstract available.
Abstract
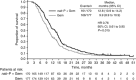
Background: This exploratory analysis evaluated second-line (2L) therapy for metastatic pancreatic cancer in a large phase 3 trial (MPACT).
Methods: Patients who received first-line (1L) nab-paclitaxel+gemcitabine (nab-P+Gem) or Gem were assessed for survival based on 2L treatment received. Multivariate analyses tested influence of treatment effect and prognostic factors on survival.
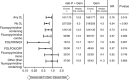
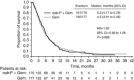
Results: The majority of 2L treatments (267 out of 347, 77%) contained a fluoropyrimidine (5-fluorouracil or capecitabine). Median total survival (1L randomisation to death) for patients who received 2L treatment after 1L nab-P+Gem vs Gem alone was 12.8 vs 9.9 months (P=0.015). Median total survival for patients with a fluoropyrimidine-containing 2L therapy after nab-P+Gem vs Gem was 13.5 vs 9.5 months (P=0.012). Median 2L survival (duration from start of 2L therapy to death) was 5.3 vs 4.5 months for nab-P+Gem vs Gem, respectively (P=0.886). Factors significantly associated with longer post-1L survival by multivariate analyses included 1L nab-P+Gem, receiving 2L treatment, longer 1L progression-free survival, and Karnofsky performance status⩾70 and neutrophil-to-lymphocyte ratio⩽5 at the end of 1L treatment.
Conclusions: These findings support the use of 2L therapy for patients with metastatic pancreatic cancer. Fluoropyrimidine-containing treatment after 1L nab-P+Gem is an active regimen with significant clinical effect.
Conflict of interest statement
EGC: research funding, consultant or advisory role, Celgene. DDVH: consultant or advisory role, honoraria, Celgene; research funding, HonorHealth. JT: consultant or advisory role, honoraria, Celgene. RE-M: consultant or advisory role, research funding, Celgene. WWM: research funding, Celgene. MR: consultant or advisory role, honoraria, research funding, Celgene. MH and RW: nothing to disclose. HL, JSL, VM, AR and BL: employment, stock ownership, Celgene. DG: research funding, Celgene, Pfizer; consultant or advisory role (unremunerated), Celgene, Pfizer.
Figures
References
-
- Abrams TA, Meyer G, Moloney J, Meyerhardt JA, Wolpin BW, Schrag D, Fuchs CS (2014) Patterns of chemotherapy (CT) use in a population-based US-wide cohort of patients (pts) with metastatic pancreatic cancer (MPC). J Clin Oncol 32(suppl 5): abstract 4131.
-
- American Cancer Society (2015) Cancer Facts and Figures 2015. American Cancer Society: Atlanta, GA, USA.
-
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413. - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364: 1817–1825. - PubMed
-
- Gill S, Ko Y, Cripps C, Beaudoin A, Dhesy-Thind SK, Zulfiqar M, Salweski P, Do T, Cano PO, Lam W, Dowden SD, Grassin H, Stewart J, Moore MJ (2014) PANCREOX: a randomized phase 3 study of 5FU/LV with or without oxaliplatin for second-line advanced pancreatic cancer (APC) in patients (pts) who have received gemcitabine (GEM)-based chemotherapy (CT). J Clin Oncol 32(suppl 5): abstract 4022. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

