Transmetalation Process as a Route for Preparation of Zinc-Oxide-Supported Copper Nanoparticles
- PMID: 27351220
- PMCID: PMC5094711
- DOI: 10.1021/acs.langmuir.6b00061
Transmetalation Process as a Route for Preparation of Zinc-Oxide-Supported Copper Nanoparticles
Abstract
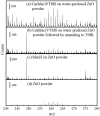
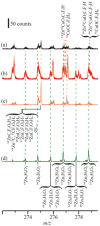
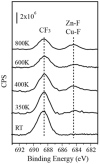
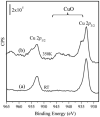
Supported nanoparticulate materials have a variety of uses, from energy storage to catalysis. In preparing such materials, precision control can often be achieved by applying chemical deposition methods. However, ligand removal following the initial deposition presents a substantial challenge because of potential surface contamination. Traditional approaches normally include multistep processing and require a substantial thermal budget. Using transmetalation chemistry, it is possible to circumvent both disadvantages and prepare chemically reactive copper nanoparticles supported on a commercially available ZnO powder material by metalorganic vapor copper deposition followed by very mild annealing to 350 K. The self-limiting copper deposition reaction is used to demonstrate the utility of this approach for hexafluoroacetylacetonate-copper-vinyltrimethylsilane, Cu(hfac)VTMS, reacting with ZnO. The low-temperature transmetalation is confirmed by a combination of spectroscopic studies. Model density functional theory calculations are consistent with a thermodynamic driving force for the process.
Figures




References
-
- Henry CR. Surface Studies of Supported Model Catalysts. Surf Sci Rep. 1998;31:231–325.
-
- Stakheev AY, Kustov LM. Effects of the Support on the Morphology and Electronic Properties of Supported Metal Clusters: Modern Concepts and Progress in 1990s. Appl Catal, A. 1999;188:3–35.
-
- George SM. Atomic Layer Deposition: An Overview. Chem Rev. 2010;110:111–131. - PubMed
-
- Cuenya BR. Synthesis and Catalytic Properties of Metal Nanoparticles: Size, Shape, Support, Composition, and Oxidation State Effects. Thin Solid Films. 2010;518:3127–3150.
-
- Campelo JM, Luna D, Luque R, Marinas JM, Romero AA. Sustainable Preparation of Supported Metal Nanoparticles and Their Applications in Catalysis. ChemSusChem. 2009;2:18–45. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

