Long-Term Use of Everolimus in Patients with Tuberous Sclerosis Complex: Final Results from the EXIST-1 Study
- PMID: 27351628
- PMCID: PMC4924870
- DOI: 10.1371/journal.pone.0158476
Long-Term Use of Everolimus in Patients with Tuberous Sclerosis Complex: Final Results from the EXIST-1 Study
Abstract
Background: Everolimus, a mammalian target of rapamycin (mTOR) inhibitor, has demonstrated efficacy in treating subependymal giant cell astrocytomas (SEGAs) and other manifestations of tuberous sclerosis complex (TSC). However, long-term use of mTOR inhibitors might be necessary. This analysis explored long-term efficacy and safety of everolimus from the conclusion of the EXIST-1 study (NCT00789828).
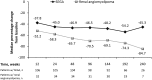
Methods and findings: EXIST-1 was an international, prospective, double-blind, placebo-controlled phase 3 trial examining everolimus in patients with new or growing TSC-related SEGA. After a double-blind core phase, all remaining patients could receive everolimus in a long-term, open-label extension. Everolimus was initiated at a dose (4.5 mg/m2/day) titrated to a target blood trough of 5-15 ng/mL. SEGA response rate (primary end point) was defined as the proportion of patients achieving confirmed ≥50% reduction in the sum volume of target SEGA lesions from baseline in the absence of worsening nontarget SEGA lesions, new target SEGA lesions, and new or worsening hydrocephalus. Of 111 patients (median age, 9.5 years) who received ≥1 dose of everolimus (median duration, 47.1 months), 57.7% (95% confidence interval [CI], 47.9-67.0) achieved SEGA response. Of 41 patients with target renal angiomyolipomas at baseline, 30 (73.2%) achieved renal angiomyolipoma response. In 105 patients with ≥1 skin lesion at baseline, skin lesion response rate was 58.1%. Incidence of adverse events (AEs) was comparable with that of previous reports, and occurrence of emergent AEs generally decreased over time. The most common AEs (≥30% incidence) suspected to be treatment-related were stomatitis (43.2%) and mouth ulceration (32.4%).
Conclusions: Everolimus use led to sustained reduction in tumor volume, and new responses were observed for SEGA and renal angiomyolipoma from the blinded core phase of the study. These findings support the hypothesis that everolimus can safely reverse multisystem manifestations of TSC in a significant proportion of patients.
Trial registration: ClinicalTrials.gov NCT00789828.
Conflict of interest statement
Figures


References
-
- Budde K, Gaedeke J. Tuberous sclerosis complex—associated angiomyolipomas: focus on mTOR inhibition. Am J Kidney Dis. 2012; 59(2):276–283. - PubMed
-
- Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991; 615:125–127. - PubMed
-
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006; 355(13):1345–1356. - PubMed
-
- Goh S, Butler W, Thiele EA. Subependymal giant cell tumors in tuberous sclerosis complex. Neurology. 2004; 63(8):1457–1461. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

