Fructose Promotes Uptake and Activity of Oligonucleotides With Different Chemistries in a Context-dependent Manner in mdx Mice
- PMID: 27351681
- PMCID: PMC5022132
- DOI: 10.1038/mtna.2016.46
Fructose Promotes Uptake and Activity of Oligonucleotides With Different Chemistries in a Context-dependent Manner in mdx Mice
Erratum in
-
Erratum: Fructose Promotes Uptake and Activity of Oligonucleotides with Different Chemistries in a Context-Dependent Manner in mdx Mice.Mol Ther Nucleic Acids. 2020 Nov 19;22:1040-1042. doi: 10.1016/j.omtn.2020.08.028. eCollection 2020 Dec 4. Mol Ther Nucleic Acids. 2020. PMID: 33294290 Free PMC article.
Abstract
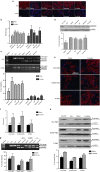
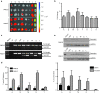
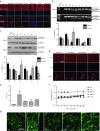
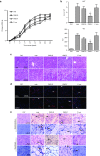
Antisense oligonucleotide (AO)-mediated exon-skipping therapeutics shows great promise in correcting frame-disrupting mutations in the DMD gene for Duchenne muscular dystrophy. However, insufficient systemic delivery limits clinical adoption. Previously, we showed that a glucose/fructose mixture augmented AO delivery to muscle in mdx mice. Here, we evaluated if fructose alone could enhance the activities of AOs with different chemistries in mdx mice. The results demonstrated that fructose improved the potency of AOs tested with the greatest effect on phosphorodiamidate morpholino oligomer (PMO), resulted in a 4.25-fold increase in the number of dystrophin-positive fibres, compared to PMO in saline in mdx mice. Systemic injection of lissamine-labeled PMO with fructose at 25 mg/kg led to increased uptake and elevated dystrophin expression in peripheral muscles, compared to PMO in saline, suggesting that fructose potentiates PMO by enhancing uptake. Repeated intravenous administration of PMO in fructose at 50 mg/kg/week for 3 weeks and 50 mg/kg/month for 5 months restored up to 20% of wild-type dystrophin levels in skeletal muscles with improved functions without detectable toxicity, compared to untreated mdx controls. Collectively, we show that fructose can potentiate AOs of different chemistries in vivo although the effect diminished over repeated administration.
Figures

Similar articles
-
MOTS-c promotes phosphorodiamidate morpholino oligomer uptake and efficacy in dystrophic mice.EMBO Mol Med. 2021 Feb 5;13(2):e12993. doi: 10.15252/emmm.202012993. Epub 2020 Dec 18. EMBO Mol Med. 2021. PMID: 33337582 Free PMC article.
-
Hexose Potentiates Peptide-Conjugated Morpholino Oligomer Efficacy in Cardiac Muscles of Dystrophic Mice in an Age-Dependent Manner.Mol Ther Nucleic Acids. 2019 Dec 6;18:341-350. doi: 10.1016/j.omtn.2019.09.012. Epub 2019 Sep 23. Mol Ther Nucleic Acids. 2019. PMID: 31629961 Free PMC article.
-
Bubble liposomes and ultrasound exposure improve localized morpholino oligomer delivery into the skeletal muscles of dystrophic mdx mice.Mol Pharm. 2014 Mar 3;11(3):1053-61. doi: 10.1021/mp4004755. Epub 2014 Jan 27. Mol Pharm. 2014. PMID: 24433046
-
Long-Term Morpholino Oligomers in Hexose Elicits Long-Lasting Therapeutic Improvements in mdx Mice.Mol Ther Nucleic Acids. 2018 Sep 7;12:478-489. doi: 10.1016/j.omtn.2018.06.005. Epub 2018 Jun 21. Mol Ther Nucleic Acids. 2018. PMID: 30195785 Free PMC article.
-
Immortalized Muscle Cell Model to Test the Exon Skipping Efficacy for Duchenne Muscular Dystrophy.J Pers Med. 2017 Oct 16;7(4):13. doi: 10.3390/jpm7040013. J Pers Med. 2017. PMID: 29035327 Free PMC article. Review.
Cited by
-
Polyquaternium-mediated delivery of morpholino oligonucleotides for exon-skipping in vitro and in mdx mice.Drug Deliv. 2017 Nov;24(1):952-961. doi: 10.1080/10717544.2017.1337827. Drug Deliv. 2017. PMID: 28633548 Free PMC article.
-
Aminoglycoside Enhances the Delivery of Antisense Morpholino Oligonucleotides In Vitro and in mdx Mice.Mol Ther Nucleic Acids. 2019 Jun 7;16:663-674. doi: 10.1016/j.omtn.2019.04.023. Epub 2019 May 2. Mol Ther Nucleic Acids. 2019. PMID: 31121478 Free PMC article.
-
Polymeric nanoparticles functionalized with muscle-homing peptides for targeted delivery of phosphatase and tensin homolog inhibitor to skeletal muscle.Acta Biomater. 2020 Dec;118:196-206. doi: 10.1016/j.actbio.2020.10.009. Epub 2020 Oct 11. Acta Biomater. 2020. PMID: 33053428 Free PMC article.
-
Tween 85-Modified Low Molecular Weight PEI Enhances Exon-Skipping of Antisense Morpholino Oligomer In Vitro and in mdx Mice.Mol Ther Nucleic Acids. 2017 Dec 15;9:120-131. doi: 10.1016/j.omtn.2017.09.006. Epub 2017 Sep 20. Mol Ther Nucleic Acids. 2017. PMID: 29246291 Free PMC article.
-
Saponins enhance exon skipping of 2'-O-methyl phosphorothioate oligonucleotide in vitro and in vivo.Drug Des Devel Ther. 2018 Oct 31;12:3705-3715. doi: 10.2147/DDDT.S179008. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30464402 Free PMC article.
References
-
- Hoffman, EP, Fischbeck, KH, Brown, RH, Johnson, M, Medori, R, Loike, JD et al. (1988). Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N Engl J Med 318: 1363–1368. - PubMed
-
- Sicinski, P, Geng, Y, Ryder-Cook, AS, Barnard, EA, Darlison, MG and Barnard, PJ (1989). The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244: 1578–1580. - PubMed
-
- Alter, J, Lou, F, Rabinowitz, A, Yin, H, Rosenfeld, J, Wilton, SD et al. (2006). Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med 12: 175–177. - PubMed
-
- Goemans, NM, Tulinius, M, van den Akker, JT, Burm, BE, Ekhart, PF, Heuvelmans, N et al. (2011). Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med 364: 1513–1522. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

