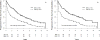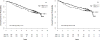Randomized phase 3 study in low-grade lymphoma comparing maintenance anti-CD20 antibody with observation after induction therapy: A trial of the ECOG-ACRIN Cancer Research Group (E1496)
- PMID: 27351685
- PMCID: PMC5030179
- DOI: 10.1002/cncr.30137
Randomized phase 3 study in low-grade lymphoma comparing maintenance anti-CD20 antibody with observation after induction therapy: A trial of the ECOG-ACRIN Cancer Research Group (E1496)
Abstract
Background: In an ECOG-ACRIN Cancer Research Group study (E1496), maintenance rituximab (MR) was reported to prolong progression-free survival (PFS) in comparison with observation (OBS) alone in patients with indolent lymphoma after induction chemotherapy. Here the long-term follow-up of the same patient cohort is presented.
Methods: Patients with indolent lymphoma received induction chemotherapy with cyclophosphamide, vincristine, and prednisone (CVP). Patients with stable disease or a better response were then randomized to weekly rituximab (375 mg/m(2) × 4 doses) every 6 months for 2 years (MR) or to OBS. The primary endpoint was PFS; the secondary endpoints were overall survival (OS), response rate, and toxicities.
Results: Of the 387 patients who initially received CVP induction, 158 were randomized to MR, and 153 were randomized to OBS. After a median follow-up of 11.5 years, patients on MR had longer median PFS (4.8 years) than patients on OBS (1.3 years; hazard ratio [HR], 0.49; P < .0001). However, there was no difference in OS between MR and OBS (10-year OS, 67% vs 59%; median OS, 13.5 years vs not reached; HR, 0.91; P = .69). Other than MR, only minimal residual disease after induction therapy was significantly associated with PFS on multivariate analysis (HR, 0.71; P = .02). A low initial tumor burden, minimal residual disease, follicular histology, a low Follicular Lymphoma International Prognostic Index score, and female sex were associated with longer OS. There was no increase in the rate of second primary malignancies with MR vs OBS.
Conclusions: With long-term follow-up, MR did not influence OS. The PFS benefit was maintained. MR should be considered optional for patients with indolent B-cell lymphoma. Cancer 2016;122:2996-3004. © 2016 American Cancer Society.
Keywords: follicular lymphoma; indolent lymphoma; non-Hodgkin lymphoma; rituximab; rituximab maintenance.
© 2016 American Cancer Society.
Conflict of interest statement
disclosures: S.H. is an employee of Genentech; the authors report no other relevant conflicts of interests
Figures
References
-
- McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–2833. - PubMed
-
- Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–4586. - PubMed
-
- Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–3732. - PubMed
-
- Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104(10):3064–3071. - PubMed
-
- Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23(33):8447–8452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA180826/CA/NCI NIH HHS/United States
- U10 CA027525/CA/NCI NIH HHS/United States
- U10 CA013650/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA017145/CA/NCI NIH HHS/United States
- U10 CA021076/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA180799/CA/NCI NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
- U10 CA180816/CA/NCI NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- U10 CA180854/CA/NCI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- U10 CA180833/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources