Reproducibility measurement of glutathione, GABA, and glutamate: Towards in vivo neurochemical profiling of multiple sclerosis with MR spectroscopy at 7T
- PMID: 27351712
- PMCID: PMC5167659
- DOI: 10.1002/jmri.25356
Reproducibility measurement of glutathione, GABA, and glutamate: Towards in vivo neurochemical profiling of multiple sclerosis with MR spectroscopy at 7T
Abstract
Purpose: To determine the reproducibility of a comprehensive single-session measurement of glutathione (GSH), γ-aminobutyric acid (GABA), glutamate, and other biochemicals implicated in the pathophysiology of multiple sclerosis (MS) in the human brain with 1 H magnetic resonance spectroscopy (MRS).
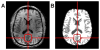
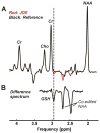
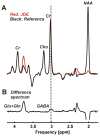
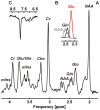
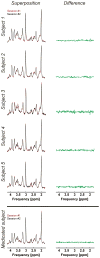
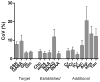
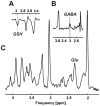
Materials and methods: Five healthy subjects were studied twice in separate 1-hour sessions at 7T. One MS patient was also scanned once. GSH and GABA were measured with J-difference editing using a semilocalized by adiabatic selective refocusing sequence (semi-LASER, TE = 72 msec). A stimulated echo acquisition mode sequence (STEAM, TE = 10 msec) was used to detect glutamate along with the overall biochemical profile. Spectra were quantified with LCModel. Quantification accuracy was assessed through Cramer-Rao lower bounds (CRLB). Reproducibility of the metabolite quantification was tested using coefficients of variation (CoV).
Results: CRLB were ≤7% for GSH, GABA, and glutamate and average CoV of 7.8 ± 3.2%, 9.5 ± 7.0%, and 3.2 ± 1.7% were achieved, respectively. The average test/retest concentration differences at this measurement reproducibility and quantification accuracy were smaller for GABA and glutamate than intersubject variations in metabolite content with CoV ratios of 0.6 and 0.8, respectively. As proof of principle, GSH, GABA, and glutamate were also detected in an MS patient.
Conclusion: GSH, GABA, glutamate, and other metabolites relevant in MS can be quantified at 7T with high accuracy and reproducibility in a single 1-hour session. This methodology might serve as a clinical research tool to investigate biochemical markers associated with MS.
Level of evidence: 2 J. Magn. Reson. Imaging 2017;45:187-198.
Keywords: 7 Tesla; brain; human; magnetic resonance spectroscopy; multiple sclerosis; reproducibility.
© 2016 International Society for Magnetic Resonance in Medicine.
Figures


References
-
- De Stefano N, Filippi M. MR spectroscopy in multiple sclerosis. J Neuroimaging. 2007;17(Suppl 1):31S–35S. - PubMed
-
- Arnold DL, Matthews PM, Francis G, Antel J. Proton magnetic resonance spectroscopy of human brain in vivo in the evaluation of multiple sclerosis: assessment of the load of disease. Magn Reson Med. 1990;14:154–159. - PubMed
-
- Davie CA, Hawkins CP, Barker GJ, et al. Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain. 1994;117(Pt 1):49–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

