Histological evaluation of a chronically-implanted electrocorticographic electrode grid in a non-human primate
- PMID: 27351722
- PMCID: PMC4993459
- DOI: 10.1088/1741-2560/13/4/046019
Histological evaluation of a chronically-implanted electrocorticographic electrode grid in a non-human primate
Abstract
Objective: Electrocorticography (ECoG), used as a neural recording modality for brain-machine interfaces (BMIs), potentially allows for field potentials to be recorded from the surface of the cerebral cortex for long durations without suffering the host-tissue reaction to the extent that it is common with intracortical microelectrodes. Though the stability of signals obtained from chronically implanted ECoG electrodes has begun receiving attention, to date little work has characterized the effects of long-term implantation of ECoG electrodes on underlying cortical tissue.
Approach: We implanted and recorded from a high-density ECoG electrode grid subdurally over cortical motor areas of a Rhesus macaque for 666 d.
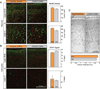
Main results: Histological analysis revealed minimal damage to the cortex underneath the implant, though the grid itself was encapsulated in collagenous tissue. We observed macrophages and foreign body giant cells at the tissue-array interface, indicative of a stereotypical foreign body response. Despite this encapsulation, cortical modulation during reaching movements was observed more than 18 months post-implantation.
Significance: These results suggest that ECoG may provide a means by which stable chronic cortical recordings can be obtained with comparatively little tissue damage, facilitating the development of clinically viable BMI systems.
Figures





References
-
- Adeeb N, Mortazavi MM, Tubbs RS, Cohen-Gadol AA. The cranial dura mater: a review of its history, embryology, and anatomy. Child’s Nervous System. 2012;28(6):827–837. - PubMed
-
- Anderson JM. Biological Response to Materials. Annual Review of Materials Research. 2001;31:81–100.
-
- Ashmore R, Endler B, Smalianchuk I, Degenhart A, Hatsopoulos N, Tyler-Kabara E, Batista A, Wang W. Stable Online Control of an Electrocorticographic Brain-Computer Interface Using a Static Decoder. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1740–1744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
