Agreements between Industry and Academia on Publication Rights: A Retrospective Study of Protocols and Publications of Randomized Clinical Trials
- PMID: 27352244
- PMCID: PMC4924795
- DOI: 10.1371/journal.pmed.1002046
Agreements between Industry and Academia on Publication Rights: A Retrospective Study of Protocols and Publications of Randomized Clinical Trials
Abstract
Background: Little is known about publication agreements between industry and academic investigators in trial protocols and the consistency of these agreements with corresponding statements in publications. We aimed to investigate (i) the existence and types of publication agreements in trial protocols, (ii) the completeness and consistency of the reporting of these agreements in subsequent publications, and (iii) the frequency of co-authorship by industry employees.
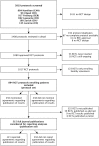
Methods and findings: We used a retrospective cohort of randomized clinical trials (RCTs) based on archived protocols approved by six research ethics committees between 13 January 2000 and 25 November 2003. Only RCTs with industry involvement were eligible. We investigated the documentation of publication agreements in RCT protocols and statements in corresponding journal publications. Of 647 eligible RCT protocols, 456 (70.5%) mentioned an agreement regarding publication of results. Of these 456, 393 (86.2%) documented an industry partner's right to disapprove or at least review proposed manuscripts; 39 (8.6%) agreements were without constraints of publication. The remaining 24 (5.3%) protocols referred to separate agreement documents not accessible to us. Of those 432 protocols with an accessible publication agreement, 268 (62.0%) trials were published. Most agreements documented in the protocol were not reported in the subsequent publication (197/268 [73.5%]). Of 71 agreements reported in publications, 52 (73.2%) were concordant with those documented in the protocol. In 14 of 37 (37.8%) publications in which statements suggested unrestricted publication rights, at least one co-author was an industry employee. In 25 protocol-publication pairs, author statements in publications suggested no constraints, but 18 corresponding protocols documented restricting agreements.
Conclusions: Publication agreements constraining academic authors' independence are common. Journal articles seldom report on publication agreements, and, if they do, statements can be discrepant with the trial protocol.
Conflict of interest statement
The authors have declared that no competing interests exist.