Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI
- PMID: 27352622
- PMCID: PMC5198282
- DOI: 10.1681/ASN.2015121376
Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI
Abstract
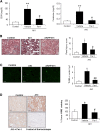
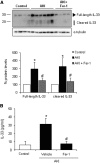
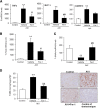
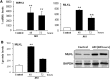
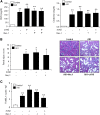
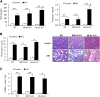
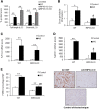
AKI is histologically characterized by necrotic cell death and inflammation. Diverse pathways of regulated necrosis have been reported to contribute to AKI, but the molecular regulators involved remain unclear. We explored the relative contributions of ferroptosis and necroptosis to folic acid (FA)-induced AKI in mice. FA-AKI in mice associates with lipid peroxidation and downregulation of glutathione metabolism proteins, features that are typical of ferroptotic cell death. We show that ferrostatin-1 (Fer-1), an inhibitor of ferroptosis, preserved renal function and decreased histologic injury, oxidative stress, and tubular cell death in this model. With respect to the immunogenicity of ferroptosis, Fer-1 prevented the upregulation of IL-33, an alarmin linked to necroptosis, and other chemokines and cytokines and prevented macrophage infiltration and Klotho downregulation. In contrast, the pancaspase inhibitor zVAD-fmk did not protect against FA-AKI. Additionally, although FA-AKI resulted in increased protein expression of the necroptosis mediators receptor-interacting protein kinase 3 (RIPK3) and mixed lineage domain-like protein (MLKL), targeting necroptosis with the RIPK1 inhibitor necrostatin-1 or genetic deficiency of RIPK3 or MLKL did not preserve renal function. Indeed, compared with wild-type mice, MLKL knockout mice displayed more severe AKI. However, RIPK3 knockout mice with AKI had less inflammation than their wild-type counterparts, and this effect associated with higher IL-10 concentration and regulatory T cell-to-leukocyte ratio in RIPK3 knockout mice. These data suggest that ferroptosis is the primary cause of FA-AKI and that immunogenicity secondary to ferroptosis may further worsen the damage, although necroptosis-related proteins may have additional roles in AKI.
Keywords: IL-33; acute renal failure; cell death; ferroptosis; inflammation; renal proximal tubule cell.
Copyright © 2016 by the American Society of Nephrology.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

