Rituximab for Severe Membranous Nephropathy: A 6-Month Trial with Extended Follow-Up
- PMID: 27352623
- PMCID: PMC5198292
- DOI: 10.1681/ASN.2016040449
Rituximab for Severe Membranous Nephropathy: A 6-Month Trial with Extended Follow-Up
Abstract
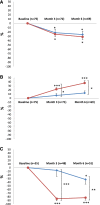
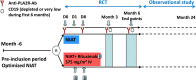
Randomized trials of rituximab in primary membranous nephropathy (PMN) have not been conducted. We undertook a multicenter, randomized, controlled trial at 31 French hospitals (NCT01508468). Patients with biopsy-proven PMN and nephrotic syndrome after 6 months of nonimmunosuppressive antiproteinuric treatment (NIAT) were randomly assigned to 6-month therapy with NIAT and 375 mg/m2 intravenous rituximab on days 1 and 8 (n=37) or NIAT alone (n=38). Median times to last follow-up were 17.0 (interquartile range, 12.5-24.0) months and 17.0 (interquartile range, 13.0-23.0) months in NIAT-rituximab and NIAT groups, respectively. Primary outcome was a combined end point of complete or partial remission of proteinuria at 6 months. At month 6, 13 (35.1%; 95% confidence interval [95% CI], 19.7 to 50.5) patients in the NIAT-rituximab group and eight (21.1%; 95% CI, 8.1 to 34.0) patients in the NIAT group achieved remission (P=0.21). Rates of antiphospholipase A2 receptor antibody (anti-PLA2R-Ab) depletion in NIAT-rituximab and NIAT groups were 14 of 25 (56%) and one of 23 (4.3%) patients at month 3 (P<0.001) and 13 of 26 (50%) and three of 25 (12%) patients at month 6 (P=0.004), respectively. Eight serious adverse events occurred in each group. During the observational phase, remission rates before change of assigned treatment were 24 of 37 (64.9%) and 13 of 38 (34.2%) patients in NIAT-rituximab and NIAT groups, respectively (P<0.01). Positive effect of rituximab on proteinuria remission occurred after 6 months. These data suggest that PLA2R-Ab levels are early markers of rituximab effect and that addition of rituximab to NIAT does not affect safety.
Keywords: anti-PLA2R antibody; anti-THSD7A antibody; idiopathic membranous nephropathy; randomized controlled trial; rituximab; severe adverse event.
Copyright © 2016 by the American Society of Nephrology.
Figures

References
-
- Hofstra JM, Fervenza FC, Wetzels JF: Treatment of idiopathic membranous nephropathy. Nat Rev Nephrol 9: 443–458, 2013 - PubMed
-
- Cravedi P, Remuzzi G, Ruggenenti P: Rituximab in primary membranous nephropathy: First-line therapy, why not? Nephron Clin Pract 128: 261–269, 2014 - PubMed
-
- Schieppati A, Mosconi L, Perna A, Mecca G, Bertani T, Garattini S, Remuzzi G: Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med 329: 85–89, 1993 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

