Use of fluorescent nanoparticles to investigate nutrient acquisition by developing Eimeria maxima macrogametocytes
- PMID: 27352801
- PMCID: PMC4926162
- DOI: 10.1038/srep29030
Use of fluorescent nanoparticles to investigate nutrient acquisition by developing Eimeria maxima macrogametocytes
Abstract
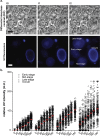
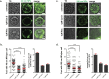
The enteric disease coccidiosis, caused by the unicellular parasite Eimeria, is a major and reoccurring problem for the poultry industry. While the molecular machinery driving host cell invasion and oocyst wall formation has been well documented in Eimeria, relatively little is known about the host cell modifications which lead to acquisition of nutrients and parasite growth. In order to understand the mechanism(s) by which nutrients are acquired by developing intracellular gametocytes and oocysts, we have performed uptake experiments using polystyrene nanoparticles (NPs) of 40 nm and 100 nm in size, as model NPs typical of organic macromolecules. Cytochalasin D and nocodazole were used to inhibit, respectively, the polymerization of the actin and microtubules. The results indicated that NPs entered the parasite at all stages of macrogametocyte development and early oocyst maturation via an active energy dependent process. Interestingly, the smaller NPs were found throughout the parasite cytoplasm, while the larger NPs were mainly localised to the lumen of large type 1 wall forming body organelles. NP uptake was reduced after microfilament disruption and treatment with nocodazole. These observations suggest that E. maxima parasites utilize at least 2 or more uptake pathways to internalize exogenous material during the sexual stages of development.
Figures



References
-
- Entzeroth R., Mattig F. R. & Werner-Meier R. Structure and function of the parasitophorous vacuole in Eimeria species. Int J Parasitol 28, 1015–1018 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

