Synthetic small molecule GLP-1 secretagogues prepared by means of a three-component indole annulation strategy
- PMID: 27352904
- PMCID: PMC4926213
- DOI: 10.1038/srep28934
Synthetic small molecule GLP-1 secretagogues prepared by means of a three-component indole annulation strategy
Abstract
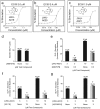
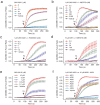
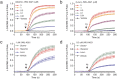
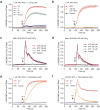
Rational assembly of small molecule libraries for purposes of drug discovery requires an efficient approach in which the synthesis of bioactive compounds is enabled so that numerous structurally related compounds of a similar basic formulation can be derived. Here, we describe (4 + 3) and (3 + 2) indole annulation strategies that quickly generate complex indole heterocycle libraries that contain novel cyclohepta- and cyclopenta[b]indoles, respectively. Screening of one such library comprised of these indoles identifies JWU-A021 to be an especially potent stimulator of glucagon-like peptide-1 (GLP-1) secretion in vitro. Surprisingly, JWU-A021 is also a potent stimulator of Ca(2+) influx through TRPA1 cation channels (EC50 ca. 200 nM), thereby explaining its ability to stimulate GLP-1 release. Of additional importance, the available evidence indicates that JWU-A021 is one of the most potent non-electrophilic TRPA-1 channel agonists yet to be reported in the literature.
Figures

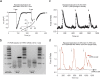
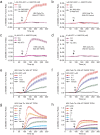
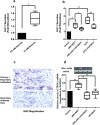
Similar articles
-
A selective small molecule glucagon-like peptide-1 secretagogue acting via depolarization-coupled Ca(2+) influx.J Endocrinol. 2009 Jun;201(3):361-7. doi: 10.1677/JOE-08-0528. Epub 2009 Mar 30. J Endocrinol. 2009. PMID: 19332449
-
Deoxynivalenol (Vomitoxin)-Induced Cholecystokinin and Glucagon-Like Peptide-1 Release in the STC-1 Enteroendocrine Cell Model Is Mediated by Calcium-Sensing Receptor and Transient Receptor Potential Ankyrin-1 Channel.Toxicol Sci. 2015 Jun;145(2):407-17. doi: 10.1093/toxsci/kfv061. Epub 2015 Mar 18. Toxicol Sci. 2015. PMID: 25787141 Free PMC article.
-
GPR119 Agonist AS1269574 Activates TRPA1 Cation Channels to Stimulate GLP-1 Secretion.Mol Endocrinol. 2016 Jun;30(6):614-29. doi: 10.1210/me.2015-1306. Epub 2016 Apr 15. Mol Endocrinol. 2016. PMID: 27082897 Free PMC article.
-
Carbohydrate-induced secretion of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1.J Diabetes Investig. 2016 Apr;7 Suppl 1(Suppl 1):27-32. doi: 10.1111/jdi.12449. Epub 2016 Mar 14. J Diabetes Investig. 2016. PMID: 27186352 Free PMC article. Review.
-
Gastrointestinal satiety signals in humans--physiologic roles for GLP-1 and PYY?Physiol Behav. 2006 Nov 30;89(4):460-4. doi: 10.1016/j.physbeh.2006.05.048. Epub 2006 Jul 7. Physiol Behav. 2006. PMID: 16828127 Review.
Cited by
-
Polygonum aviculare L. extract and quercetin attenuate contraction in airway smooth muscle.Sci Rep. 2018 Feb 15;8(1):3114. doi: 10.1038/s41598-018-20409-x. Sci Rep. 2018. PMID: 29449621 Free PMC article.
-
Semen cassiae Extract Inhibits Contraction of Airway Smooth Muscle.Front Pharmacol. 2018 Dec 4;9:1389. doi: 10.3389/fphar.2018.01389. eCollection 2018. Front Pharmacol. 2018. PMID: 30564120 Free PMC article.
-
An Overview of the TRP-Oxidative Stress Axis in Metabolic Syndrome: Insights for Novel Therapeutic Approaches.Cells. 2022 Apr 11;11(8):1292. doi: 10.3390/cells11081292. Cells. 2022. PMID: 35455971 Free PMC article. Review.
-
miR-204 Controls Glucagon-Like Peptide 1 Receptor Expression and Agonist Function.Diabetes. 2018 Feb;67(2):256-264. doi: 10.2337/db17-0506. Epub 2017 Nov 3. Diabetes. 2018. PMID: 29101219 Free PMC article.
-
Nucleophile-intercepted Beckmann fragmentation reactions.Chem Sci. 2019 Jul 4;10(33):7812-7815. doi: 10.1039/c9sc00926d. eCollection 2019 Sep 7. Chem Sci. 2019. PMID: 31588331 Free PMC article.
References
-
- Vitaku E., Smith D. T. & Njardarson J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals: miniperspective. J. Med. Chem. 57, 10257–10274 (2014). - PubMed
-
- Han X., Li H., Hughes R. P. & Wu J. Gallium(III)-catalyzed three-component (4 + 3) cycloaddition reactions. Angew. Chem. Int. Ed. 51, 10390–10393 (2012). - PubMed
-
- Nilius B., Appendino G. & Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch. 464, 425–458 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

