Structural and molecular bases of rod photoreceptor morphogenesis and disease
- PMID: 27352937
- PMCID: PMC5112133
- DOI: 10.1016/j.preteyeres.2016.06.002
Structural and molecular bases of rod photoreceptor morphogenesis and disease
Abstract
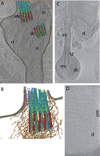
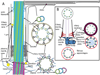
The rod cell has an extraordinarily specialized structure that allows it to carry out its unique function of detecting individual photons of light. Both the structural features of the rod and the metabolic processes required for highly amplified light detection seem to have rendered the rod especially sensitive to structural and metabolic defects, so that a large number of gene defects are primarily associated with rod cell death and give rise to blinding retinal dystrophies. The structures of the rod, especially those of the sensory cilium known as the outer segment, have been the subject of structural, biochemical, and genetic analysis for many years, but the molecular bases for rod morphogenesis and for cell death in rod dystrophies are still poorly understood. Recent developments in imaging technology, such as cryo-electron tomography and super-resolution fluorescence microscopy, in gene sequencing technology, and in gene editing technology are rapidly leading to new breakthroughs in our understanding of these questions. A summary is presented of our current understanding of selected aspects of these questions, highlighting areas of uncertainty and contention as well as recent discoveries that provide new insights. Examples of structural data from emerging imaging technologies are presented.
Keywords: Ciliopathies; Cryo-electron tomography; Disease mechanisms; Photoreceptor; Retinal degeneration; Retinal imaging.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures





References
-
- Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–125. - PubMed
-
- Aguirre G. Hereditary retinal diseases in small animals. The Veterinary clinics of North America. 1973;3:515–528. - PubMed
-
- Aguirre G, Acland G, Chader G. Hereditary retinal degenerations in the dog: specificity of abnormal cyclic nucleotide metabolism to diseases of arrested photoreceptor development. Birth defects original article series. 1982;18:119–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 EY027171/EY/NEI NIH HHS/United States
- T32 GM008280/GM/NIGMS NIH HHS/United States
- R01 EY011900/EY/NEI NIH HHS/United States
- T32 EY007001/EY/NEI NIH HHS/United States
- P30 EY002520/EY/NEI NIH HHS/United States
- F32 EY024815/EY/NEI NIH HHS/United States
- P30 EY011373/EY/NEI NIH HHS/United States
- R01 EY011731/EY/NEI NIH HHS/United States
- R01 EY007981/EY/NEI NIH HHS/United States
- P41 GM103832/GM/NIGMS NIH HHS/United States
- R01 EY025218/EY/NEI NIH HHS/United States
- R01 EY026545/EY/NEI NIH HHS/United States
- P41 RR002250/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

