Efficacy of histotripsy combined with rt-PA in vitro
- PMID: 27353199
- PMCID: PMC5563443
- DOI: 10.1088/0031-9155/61/14/5253
Efficacy of histotripsy combined with rt-PA in vitro
Abstract
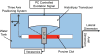
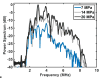
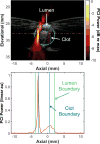
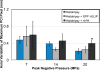
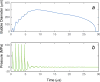
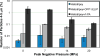
Histotripsy, a form of therapeutic ultrasound that uses the mechanical action of microbubble clouds for tissue ablation, is under development to treat chronic deep vein thrombosis (DVT). We hypothesize that combining thrombolytic agents with histotripsy will enhance clot lysis. Recombinant tissue plasminogen activator (rt-PA) and rt-PA-loaded echogenic liposomes that entrain octafluoropropane microbubbles (OFP t-ELIP) were used in combination with highly shocked histotripsy pulses. Fully retracted porcine venous clots, with similar features of DVT occlusions, were exposed either to histotripsy pulses alone (peak negative pressures of 7-20 MPa), histotripsy and OFP t-ELIP, or histotripsy and rt-PA. Microbubble cloud activity was monitored with passive cavitation imaging during histotripsy exposure. The power levels of cavitation emissions from within the clot were not statistically different between treatment types, likely due to the near instantaneous rupture and destruction of OFP t-ELIP. The thrombolytic efficacy was significantly improved in the presence of rt-PA. These results suggest the combination of histotripsy and rt-PA could serve as a potent therapeutic strategy for the treatment of DVT.
Figures










References
-
- Augustinos P. Invasive Approaches to Treatment of Venous Thromboembolism. Circulation. 2004;110:I-27–I-34. - PubMed
-
- Avgerinos ED, Hager ES, Naddaf A, Dillavou E, Singh M, Chaer RA. Outcomes and predictors of failure of thrombolysisfor iliofemoral deep venous thrombosis. J Vasc Surg. 2015;3:35–41. - PubMed
-
- Aziz F, Comerota AJ. Quantity of residual thrombus after successful catheter-directed thrombolysis for iliofemoral deep vein thrombosis correlates with recurrence. Eur J Vasc Endovasc Surg. 2012;44:210–3. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
