NLRP6 function in inflammatory monocytes reduces susceptibility to chemically induced intestinal injury
- PMID: 27353251
- PMCID: PMC5199680
- DOI: 10.1038/mi.2016.55
NLRP6 function in inflammatory monocytes reduces susceptibility to chemically induced intestinal injury
Abstract
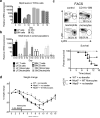
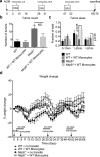
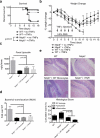
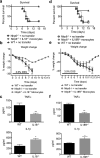
NLRP6 is a member of the Nod-like receptor family, whose members are involved in the recognition of microbes and/or tissue injury. NLRP6 was previously demonstrated to regulate the production of interleukin (IL)-18 and is important for protecting mice against chemically induced intestinal injury and colitis-associated colon cancer. However, the cellular mechanisms by which NLRP6 reduces susceptibility to colonic inflammation remain unclear. Here, we determined that NLRP6 expression is specifically upregulated in Ly6Chi inflammatory monocytes that infiltrate into the colon during dextran sulfate sodium (DSS)-induced inflammation. Adoptive transfer of wild-type (WT) Ly6Chi inflammatory monocytes into Nlrp6-/- mice was sufficient to protect them from mortality, significantly reducing intestinal permeability and damage. NLRP6-deficient inflammatory monocytes were defective in tumor necrosis factor α (TNFα) production, which was important for reducing DSS-induced mortality and was dependent on autocrine IL-18 signaling by inflammatory monocytes. Our data reveal a previously unappreciated role for NLRP6 in inflammatory monocytes, which are recruited after DSS-induced intestinal injury to promote barrier function and limit bacteria-driven inflammation. This study highlights the importance of early cytokine responses, particularly NLRP6-dependent and IL-18-dependent TNFα production, in preventing chronic dysregulated inflammation.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

