Role of Cl- -HCO3- exchanger AE3 in intracellular pH homeostasis in cultured murine hippocampal neurons, and in crosstalk to adjacent astrocytes
- PMID: 27353306
- PMCID: PMC5199729
- DOI: 10.1113/JP272470
Role of Cl- -HCO3- exchanger AE3 in intracellular pH homeostasis in cultured murine hippocampal neurons, and in crosstalk to adjacent astrocytes
Abstract
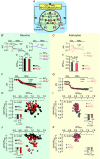
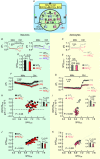
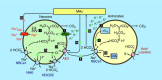
Key points: A polymorphism of human AE3 is associated with idiopathic generalized epilepsy. Knockout of AE3 in mice lowers the threshold for triggering epileptic seizures. The explanations for these effects are elusive. Comparisons of cells from wild-type vs. AE3-/- mice show that AE3 (present in hippocampal neurons, not astrocytes; mediates HCO3- efflux) enhances intracellular pH (pHi ) recovery (decrease) from alkali loads in neurons and, surprisingly, adjacent astrocytes. During metabolic acidosis (MAc), AE3 speeds initial acidification, but limits the extent of pHi decrease in neurons and astrocytes. AE3 speeds re-alkalization after removal of MAc in neurons and astrocytes, and speeds neuronal pHi recovery from an ammonium prepulse-induced acid load. We propose that neuronal AE3 indirectly increases acid extrusion in (a) neurons via Cl- loading, and (b) astrocytes by somehow enhancing NBCe1 (major acid extruder). The latter would enhance depolarization-induced alkalinization of astrocytes, and extracellular acidification, and thereby reduce susceptibility to epileptic seizures.
Abstract: The anion exchanger AE3, expressed in hippocampal (HC) neurons but not astrocytes, contributes to intracellular pH (pHi ) regulation by facilitating the exchange of extracellular Cl- for intracellular HCO3- . The human AE3 polymorphism A867D is associated with idiopathic generalized epilepsy. Moreover, AE3 knockout (AE3-/- ) mice are more susceptible to epileptic seizure. The mechanism of these effects has been unclear because the starting pHi in AE3-/- and wild-type neurons is indistinguishable. The purpose of the present study was to use AE3-/- mice to investigate the role of AE3 in pHi homeostasis in HC neurons, co-cultured with astrocytes. We find that the presence of AE3 increases the acidification rate constant during pHi recovery from intracellular alkaline loads imposed by reducing [CO2 ]. The presence of AE3 also speeds intracellular acidification during the early phase of metabolic acidosis (MAc), not just in neurons but, surprisingly, in adjacent astrocytes. Additionally, AE3 contributes to braking the decrease in pHi later during MAc in both neurons and astrocytes. Paradoxically, AE3 enhances intracellular re-alkalization after MAc removal in neurons and astrocytes, and pHi recovery from an ammonium prepulse-induced acid load in neurons. The effects of AE3 knockout on astrocytic pHi homeostasis in MAc-related assays require the presence of neurons, and are consistent with the hypothesis that the AE3 knockout reduces functional expression of astrocytic NBCe1. These findings suggest a new type of neuron-astrocyte communication, based on the expression of AE3 in neurons, which could explain how AE3 reduces seizure susceptibility.
Keywords: Slc4a3; acid-base balance; acid-extrusion rate from cells; acid-loading rate of cells; anion exchanger.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures





Comment in
-
The mysterious role of the neuronal anion exchanger-3.J Physiol. 2017 Feb 1;595(3):601-602. doi: 10.1113/JP273033. Epub 2017 Jan 17. J Physiol. 2017. PMID: 28097651 Free PMC article. No abstract available.
Similar articles
-
Mice with a targeted disruption of the Cl-/HCO3- exchanger AE3 display a reduced seizure threshold.Mol Cell Biol. 2006 Jan;26(1):182-91. doi: 10.1128/MCB.26.1.182-191.2006. Mol Cell Biol. 2006. PMID: 16354689 Free PMC article.
-
Characterization of an epilepsy-associated variant of the human Cl-/HCO3(-) exchanger AE3.Am J Physiol Cell Physiol. 2009 Sep;297(3):C526-36. doi: 10.1152/ajpcell.00572.2008. Epub 2009 Jul 15. Am J Physiol Cell Physiol. 2009. PMID: 19605733
-
Bicarbonate sensing in mouse cortical astrocytes during extracellular acid/base disturbances.J Physiol. 2017 Apr 15;595(8):2569-2585. doi: 10.1113/JP273394. Epub 2017 Feb 15. J Physiol. 2017. PMID: 27981578 Free PMC article.
-
Intracellular pH regulation by acid-base transporters in mammalian neurons.Front Physiol. 2014 Feb 13;5:43. doi: 10.3389/fphys.2014.00043. eCollection 2014. Front Physiol. 2014. PMID: 24592239 Free PMC article. Review.
-
pH regulating mechanisms of astrocytes: A critical component in physiology and disease of the brain.Cell Calcium. 2024 Jun;120:102882. doi: 10.1016/j.ceca.2024.102882. Epub 2024 Apr 8. Cell Calcium. 2024. PMID: 38631162 Free PMC article. Review.
Cited by
-
Inward Operation of Sodium-Bicarbonate Cotransporter 1 Promotes Astrocytic Na+ Loading and Loss of ATP in Mouse Neocortex during Brief Chemical Ischemia.Cells. 2023 Nov 21;12(23):2675. doi: 10.3390/cells12232675. Cells. 2023. PMID: 38067105 Free PMC article.
-
SLC4A10 mutation causes a neurological disorder associated with impaired GABAergic transmission.Brain. 2023 Nov 2;146(11):4547-4561. doi: 10.1093/brain/awad235. Brain. 2023. PMID: 37459438 Free PMC article.
-
Mouse models of SLC4-linked disorders of HCO3--transporter dysfunction.Am J Physiol Cell Physiol. 2018 May 1;314(5):C569-C588. doi: 10.1152/ajpcell.00301.2017. Epub 2018 Jan 31. Am J Physiol Cell Physiol. 2018. PMID: 29384695 Free PMC article. Review.
-
TGF-β signaling directly regulates transcription and functional expression of the electrogenic sodium bicarbonate cotransporter 1, NBCe1 (SLC4A4), via Smad4 in mouse astrocytes.Glia. 2017 Aug;65(8):1361-1375. doi: 10.1002/glia.23168. Epub 2017 Jun 1. Glia. 2017. PMID: 28568893 Free PMC article.
-
Novel RPTPγ and RPTPζ splice variants from mixed neuron-astrocyte hippocampal cultures as well as from the hippocampi of newborn and adult mice.Front Physiol. 2024 Jun 17;15:1406448. doi: 10.3389/fphys.2024.1406448. eCollection 2024. Front Physiol. 2024. PMID: 38952869 Free PMC article.
References
-
- Aharonovitz O, Kapus A, Szászi K, Coady‐Osberg N, Jancelewicz T, Orlowski J & Grinstein S (2001). Modulation of Na+/H+ exchange activity by Cl− . Am J Physiol Cell Physiol 281, C133–C141. - PubMed
-
- Arroyo JP, Kahle KT & Gamba G (2013). The SLC12 family of electroneutral cation‐coupled chloride cotransporters. Mol Aspects Med 34, 288–298. - PubMed
-
- Bergeron MJ, Gagnon E, Wallendorff B, Lapointe J‐Y & Isenring P (2003). Ammonium transport and pH regulation by K‐Cl cotransporters. Am J Physiol Renal Physiol 285, F68–F78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

