Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data
- PMID: 27353418
- PMCID: PMC4925917
- DOI: 10.1136/bmj.i3283
Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data
Abstract
Objective: To quantify how a period of intense media coverage of controversy over the risk:benefit balance of statins affected their use.
Design: Interrupted time series analysis of prospectively collected electronic data from primary care.
Setting: Clinical Practice Research Datalink (CPRD) in the United Kingdom.
Participants: Patients newly eligible for or currently taking statins for primary and secondary cardiovascular disease prevention in each month in January 2011-March 2015.
Main outcome measures: Adjusted odds ratios for starting/stopping taking statins after the media coverage (October 2013-March 2014).
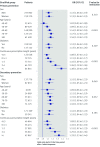
Results: There was no evidence that the period of high media coverage was associated with changes in statin initiation among patients with a high recorded risk score for cardiovascular disease (primary prevention) or a recent cardiovascular event (secondary prevention) (odds ratio 0.99 (95% confidence interval 0.87 to 1.13; P=0.92) and 1.04 (0.92 to 1.18; P=0.54), respectively), though there was a decrease in the overall proportion of patients with a recorded risk score. Patients already taking statins were more likely to stop taking them for both primary and secondary prevention after the high media coverage period (1.11 (1.05 to 1.18; P<0.001) and 1.12 (1.04 to 1.21; P=0.003), respectively). Stratified analyses showed that older patients and those with a longer continuous prescription were more likely to stop taking statins after the media coverage. In post hoc analyses, the increased rates of cessation were no longer observed after six months.
Conclusions: A period of intense public discussion over the risks:benefit balance of statins, covered widely in the media, was followed by a transient rise in the proportion of people who stopped taking statins. This research highlights the potential for widely covered health stories in the lay media to impact on healthcare related behaviour.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures



Comment in
-
Why do people not take life-saving medications? The case of statins.Lancet. 2016 Sep 3;388(10048):943-5. doi: 10.1016/S0140-6736(16)31532-X. Epub 2016 Sep 1. Lancet. 2016. PMID: 27598664 No abstract available.
References
-
- Baigent C, Blackwell L, Emberson J, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670-81. 10.1016/S0140-6736(10)61350-5 pmid:21067804. - DOI - PMC - PubMed
-
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;1:CD004816.pmid:23440795. - PMC - PubMed
-
- Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009;338:b2376 10.1136/bmj.b2376 pmid:19567909. - DOI - PMC - PubMed
-
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1423 10.1136/bmj.326.7404.1423 pmid:12829554. - DOI - PMC - PubMed
-
- Pignone M, Phillips C, Mulrow C. Use of lipid lowering drugs for primary prevention of coronary heart disease: meta-analysis of randomised trials. BMJ 2000;321:983-6. 10.1136/bmj.321.7267.983 pmid:11039962. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
