Overexpression of Plasmodium berghei ATG8 by Liver Forms Leads to Cumulative Defects in Organelle Dynamics and to Generation of Noninfectious Merozoites
- PMID: 27353755
- PMCID: PMC4937212
- DOI: 10.1128/mBio.00682-16
Overexpression of Plasmodium berghei ATG8 by Liver Forms Leads to Cumulative Defects in Organelle Dynamics and to Generation of Noninfectious Merozoites
Abstract
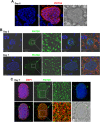
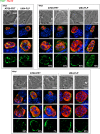
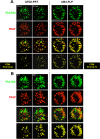
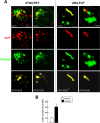
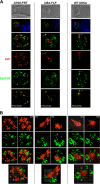
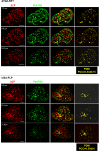
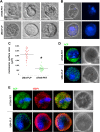
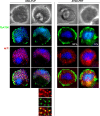
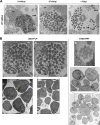
Plasmodium parasites undergo continuous cellular renovation to adapt to various environments in the vertebrate host and insect vector. In hepatocytes, Plasmodium berghei discards unneeded organelles for replication, such as micronemes involved in invasion. Concomitantly, intrahepatic parasites expand organelles such as the apicoplast that produce essential metabolites. We previously showed that the ATG8 conjugation system is upregulated in P. berghei liver forms and that P. berghei ATG8 (PbATG8) localizes to the membranes of the apicoplast and cytoplasmic vesicles. Here, we focus on the contribution of PbATG8 to the organellar changes that occur in intrahepatic parasites. We illustrated that micronemes colocalize with PbATG8-containing structures before expulsion from the parasite. Interference with PbATG8 function by overexpression results in poor development into late liver stages and production of small merosomes that contain immature merozoites unable to initiate a blood infection. At the cellular level, PbATG8-overexpressing P. berghei exhibits a delay in microneme compartmentalization into PbATG8-containing autophagosomes and elimination compared to parasites from the parental strain. The apicoplast, identifiable by immunostaining of the acyl carrier protein (ACP), undergoes an abnormally fast proliferation in mutant parasites. Over time, the ACP staining becomes diffuse in merosomes, indicating a collapse of the apicoplast. PbATG8 is not incorporated into the progeny of mutant parasites, in contrast to parental merozoites in which PbATG8 and ACP localize to the apicoplast. These observations reveal that Plasmodium ATG8 is a key effector in the development of merozoites by controlling microneme clearance and apicoplast proliferation and that dysregulation in ATG8 levels is detrimental for malaria infectivity.
Importance: Malaria is responsible for more mortality than any other parasitic disease. Resistance to antimalarial medicines is a recurring problem; new drugs are urgently needed. A key to the parasite's successful intracellular development in the liver is the metabolic changes necessary to convert the parasite from a sporozoite to a replication-competent, metabolically active trophozoite form. Our study reinforces the burgeoning concept that organellar changes during parasite differentiation are mediated by an autophagy-like process. We have identified ATG8 in Plasmodium liver forms as an important effector that controls the development and fate of organelles, e.g., the clearance of micronemes that are required for hepatocyte invasion and the expansion of the apicoplast that produces many metabolites indispensable for parasite replication. Given the unconventional properties and the importance of ATG8 for parasite development in hepatocytes, targeting the parasite's autophagic pathway may represent a novel approach to control malarial infections.
Copyright © 2016 Voss et al.
Figures

Similar articles
-
Autophagy protein Atg7 is essential for maintaining malaria parasite cellular homeostasis and organelle biogenesis.mBio. 2025 Feb 5;16(2):e0273524. doi: 10.1128/mbio.02735-24. Epub 2024 Dec 23. mBio. 2025. PMID: 39714137 Free PMC article.
-
Characterization of the ATG8-conjugation system in 2 Plasmodium species with special focus on the liver stage: possible linkage between the apicoplastic and autophagic systems?Autophagy. 2014 Feb;10(2):269-84. doi: 10.4161/auto.27166. Epub 2013 Dec 12. Autophagy. 2014. PMID: 24342964 Free PMC article.
-
The Plasmodium GRASP Homolog Modulates Liver Stage Development, Subsequent Blood Infection and Virulence in Mice.Mol Microbiol. 2025 Jun;123(6):487-515. doi: 10.1111/mmi.15360. Epub 2025 Mar 26. Mol Microbiol. 2025. PMID: 40143578
-
Metamorphoses of malaria: the role of autophagy in parasite differentiation.Essays Biochem. 2011;51:127-36. doi: 10.1042/bse0510127. Essays Biochem. 2011. PMID: 22023446 Free PMC article. Review.
-
The malaria sporozoite's journey into the liver.Infect Agents Dis. 1996 Jun;5(3):182-9. Infect Agents Dis. 1996. PMID: 8805080 Review.
Cited by
-
Post-Translational Modifications of Proteins of Malaria Parasites during the Life Cycle.Int J Mol Sci. 2024 Jun 2;25(11):6145. doi: 10.3390/ijms25116145. Int J Mol Sci. 2024. PMID: 38892332 Free PMC article. Review.
-
ATG8 Is Essential Specifically for an Autophagy-Independent Function in Apicoplast Biogenesis in Blood-Stage Malaria Parasites.mBio. 2018 Jan 2;9(1):e02021-17. doi: 10.1128/mBio.02021-17. mBio. 2018. PMID: 29295911 Free PMC article.
-
Chemoprophylaxis vaccination with a Plasmodium liver stage autophagy mutant affords enhanced and long-lasting protection.NPJ Vaccines. 2021 Aug 10;6(1):98. doi: 10.1038/s41541-021-00360-1. NPJ Vaccines. 2021. PMID: 34376691 Free PMC article.
-
Oligomerization of Selective Autophagy Receptors for the Targeting and Degradation of Protein Aggregates.Cells. 2021 Aug 5;10(8):1989. doi: 10.3390/cells10081989. Cells. 2021. PMID: 34440758 Free PMC article. Review.
-
Progressive heterogeneity of enlarged and irregularly shaped apicoplasts in P. falciparum persister blood stages after drug treatment.bioRxiv [Preprint]. 2024 Aug 29:2024.01.03.574077. doi: 10.1101/2024.01.03.574077. bioRxiv. 2024. Update in: PNAS Nexus. 2024 Sep 24;3(10):pgae424. doi: 10.1093/pnasnexus/pgae424. PMID: 38410435 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

