Cyclic diguanylate signaling in Gram-positive bacteria
- PMID: 27354347
- PMCID: PMC5007281
- DOI: 10.1093/femsre/fuw013
Cyclic diguanylate signaling in Gram-positive bacteria
Abstract
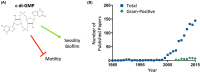
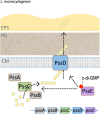
The nucleotide second messenger 3'-5' cyclic diguanylate monophosphate (c-di-GMP) is a central regulator of the transition between motile and non-motile lifestyles in bacteria, favoring sessility. Most research investigating the functions of c-di-GMP has focused on Gram-negative species, especially pathogens. Recent work in Gram-positive species has revealed that c-di-GMP plays similar roles in Gram-positives, though the precise targets and mechanisms of regulation may differ. The majority of bacterial life exists in a surface-associated state, with motility allowing bacteria to disseminate and colonize new environments. c-di-GMP signaling regulates flagellum biosynthesis and production of adherence factors and appears to be a primary mechanism by which bacteria sense and respond to surfaces. Ultimately, c-di-GMP influences the ability of a bacterium to alter its transcriptional program, physiology and behavior upon surface contact. This review discusses how bacteria are able to sense a surface via flagella and type IV pili, and the role of c-di-GMP in regulating the response to surfaces, with emphasis on studies of Gram-positive bacteria.
Keywords: Gram-positive; adherence; biofilm; cyclic diguanylate; motility; signaling.
© FEMS 2016. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Abee T, Kovacs AT, Kuipers OP, et al. Biofilm formation and dispersal in Gram-positive bacteria. Curr Opin Biotechnol. 2011;22:172–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

