The role of MyD88- and TRIF-dependent signaling in monophosphoryl lipid A-induced expansion and recruitment of innate immunocytes
- PMID: 27354411
- PMCID: PMC5109999
- DOI: 10.1189/jlb.1A0216-072R
The role of MyD88- and TRIF-dependent signaling in monophosphoryl lipid A-induced expansion and recruitment of innate immunocytes
Abstract
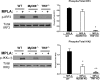
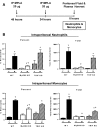
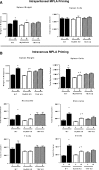
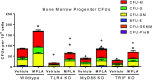
Treatment with the TLR4 agonist MPLA augments innate resistance to common bacterial pathogens. However, the cellular and molecular mechanisms by which MPLA augments innate immunocyte functions are not well characterized. This study examined the importance of MyD88- and TRIF-dependent signaling for leukocyte mobilization, recruitment, and activation following administration of MPLA. MPLA potently induced MyD88- and TRIF-dependent signaling. A single injection of MPLA caused rapid mobilization and recruitment of neutrophils, a response that was largely mediated by the chemokines CXCL1 and -2 and the hemopoietic factor G-CSF. Rapid neutrophil recruitment and chemokine production were regulated by both pathways although the MyD88-dependent pathway showed some predominance. In further studies, multiple injections of MPLA potently induced mobilization and recruitment of neutrophils and monocytes. Neutrophil recruitment after multiple injections of MPLA was reliant on MyD88-dependent signaling, but effective monocyte recruitment required activation of both pathways. MPLA treatment induced expansion of myeloid progenitors in bone marrow and upregulation of CD11b and shedding of L-selectin by neutrophils, all of which were attenuated in MyD88- and TRIF-deficient mice. These results show that MPLA-induced neutrophil and monocyte recruitment, expansion of bone marrow progenitors and augmentation of neutrophil adhesion molecule expression are regulated by both the MyD88- and TRIF-dependent pathways.
Keywords: TLR4; cytokines; innate immunity; monocyte; myeloid progenitors; neutrophil.
© Society for Leukocyte Biology.
Figures



References
-
- Qureshi N., Takayama K., Ribi E. (1982) Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J. Biol. Chem. 257, 11808–11815. - PubMed
-
- Bentala H., Verweij W. R., Huizinga-Van der Vlag A., van Loenen-Weemaes A. M., Meijer D. K., Poelstra K. (2002) Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock 18, 561–566. - PubMed
-
- Pichichero M. E. (2008) Improving vaccine delivery using novel adjuvant systems. Hum. Vaccin. 4, 262–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

