Identification of Signaling Pathways by Which CD40 Stimulates Autophagy and Antimicrobial Activity against Toxoplasma gondii in Macrophages
- PMID: 27354443
- PMCID: PMC4995900
- DOI: 10.1128/IAI.00101-16
Identification of Signaling Pathways by Which CD40 Stimulates Autophagy and Antimicrobial Activity against Toxoplasma gondii in Macrophages
Abstract
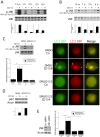
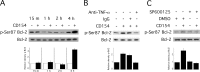
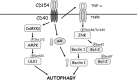
CD40 is an important stimulator of autophagy and autophagic killing of Toxoplasma gondii in host cells. In contrast to autophagy induced by nutrient deprivation or pattern recognition receptors, less is known about the effects of cell-mediated immunity on Beclin 1 and ULK1, key regulators of autophagy. Here we studied the molecular mechanisms by which CD40 stimulates autophagy in macrophages. CD40 ligation caused biphasic Jun N-terminal protein kinase (JNK) phosphorylation. The second phase of JNK phosphorylation was dependent on autocrine production of tumor necrosis factor alpha (TNF-α). TNF-α and JNK signaling were required for the CD40-induced increase in autophagy. JNK signaling downstream of CD40 caused Ser-87 phosphorylation of Bcl-2 and dissociation between Bcl-2 and Beclin 1, an event known to stimulate the autophagic function of Beclin 1. However, TNF-α alone was unable to stimulate autophagy. CD40 also stimulated autophagy via a pathway that included calcium/calmodulin-dependent kinase kinase β (CaMKKβ), AMP-activated protein kinase (AMPK), and ULK1. CD40 caused AMPK phosphorylation at its activating site, Thr-172. This effect was mediated by CaMKKβ and was not impaired by neutralization of TNF-α. CD40 triggered AMPK-dependent Ser-555 phosphorylation of ULK1. CaMKKβ, AMPK, and ULK1 were required for CD40-induced increase in autophagy. CD40-mediated autophagic killing of Toxoplasma gondii is known to require TNF-α. Knockdown of JNK, CaMKKβ, AMPK, or ULK1 prevented T. gondii killing in CD40-activated macrophages. The second phase of JNK phosphorylation-Bcl-2 phosphorylation-Bcl-2-Beclin 1 dissociation and AMPK phosphorylation-ULK1 phosphorylation occurred simultaneously at ∼4 h post-CD40 stimulation. Thus, CaMKKβ and TNF-α are upstream molecules by which CD40 acts on ULK1 and Beclin 1 to stimulate autophagy and killing of T. gondii.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




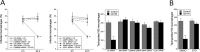
Similar articles
-
The CD40-autophagy pathway is needed for host protection despite IFN-Γ-dependent immunity and CD40 induces autophagy via control of P21 levels.PLoS One. 2010 Dec 31;5(12):e14472. doi: 10.1371/journal.pone.0014472. PLoS One. 2010. PMID: 21217818 Free PMC article.
-
CD40-TRAF6 and autophagy-dependent anti-microbial activity in macrophages.Autophagy. 2007 May-Jun;3(3):245-8. doi: 10.4161/auto.3717. Epub 2007 May 15. Autophagy. 2007. PMID: 17224624
-
The GST-BHMT assay reveals a distinct mechanism underlying proteasome inhibition-induced macroautophagy in mammalian cells.Autophagy. 2015;11(5):812-32. doi: 10.1080/15548627.2015.1034402. Autophagy. 2015. PMID: 25984893 Free PMC article.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Role of an intrinsically disordered conformation in AMPK-mediated phosphorylation of ULK1 and regulation of autophagy.Mol Biosyst. 2012 Jan;8(1):91-6. doi: 10.1039/c1mb05265a. Epub 2011 Aug 18. Mol Biosyst. 2012. PMID: 21853163 Review.
Cited by
-
Interplay Between Toxoplasma gondii, Autophagy, and Autophagy Proteins.Front Cell Infect Microbiol. 2019 May 1;9:139. doi: 10.3389/fcimb.2019.00139. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31119109 Free PMC article. Review.
-
The Interplay of Host Autophagy and Eukaryotic Pathogens.Front Cell Dev Biol. 2018 Sep 13;6:118. doi: 10.3389/fcell.2018.00118. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30271774 Free PMC article. Review.
-
Strategies Developed by Toxoplasma gondii to Survive in the Host.Front Microbiol. 2019 Apr 26;10:899. doi: 10.3389/fmicb.2019.00899. eCollection 2019. Front Microbiol. 2019. PMID: 31080445 Free PMC article. Review.
-
Omega-3 and omega-6 polyunsaturated fatty acids and their potential therapeutic role in protozoan infections.Front Immunol. 2024 Apr 3;15:1339470. doi: 10.3389/fimmu.2024.1339470. eCollection 2024. Front Immunol. 2024. PMID: 38633251 Free PMC article. Review.
-
SIRT1 Promotes Host Protective Immunity against Toxoplasma gondii by Controlling the FoxO-Autophagy Axis via the AMPK and PI3K/AKT Signalling Pathways.Int J Mol Sci. 2022 Nov 5;23(21):13578. doi: 10.3390/ijms232113578. Int J Mol Sci. 2022. PMID: 36362370 Free PMC article.
References
-
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. 2011. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331:456–461. doi:10.1126/science.1196371. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

