Long-Term Immunity to Trypanosoma cruzi in the Absence of Immunodominant trans-Sialidase-Specific CD8+ T Cells
- PMID: 27354447
- PMCID: PMC4995909
- DOI: 10.1128/IAI.00241-16
Long-Term Immunity to Trypanosoma cruzi in the Absence of Immunodominant trans-Sialidase-Specific CD8+ T Cells
Abstract
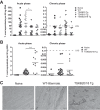
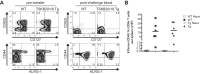
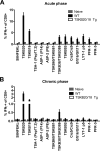
Trypanosoma cruzi infection drives the expansion of remarkably focused CD8(+) T cell responses targeting epitopes encoded by variant trans-sialidase (TS) genes. Infection of C57BL/6 mice with T. cruzi results in up to 40% of all CD8(+) T cells committed to recognition of the dominant TSKB20 and subdominant TSKB18 TS epitopes. However, despite this enormous response, these mice fail to clear T. cruzi infection and subsequently develop chronic disease. One possible reason for the failure to cure T. cruzi infection is that immunodomination by these TS-specific T cells may interfere with alternative CD8(+) T cell responses more capable of complete parasite elimination. To address this possibility, we created transgenic mice that are centrally tolerant to these immunodominant epitopes. Mice expressing TSKB20, TSKB18, or both epitopes controlled T. cruzi infection and developed effector CD8(+) T cells that maintained an activated phenotype. Memory CD8(+) T cells from drug-cured TSKB-transgenic mice rapidly responded to secondary T. cruzi infection. In the absence of the response to TSKB20 and TSKB18, immunodominance did not shift to other known subdominant epitopes despite the capacity of these mice to expand epitope-specific T cells specific for the model antigen ovalbumin expressed by engineered parasites. Thus, CD8(+) T cell responses tightly and robustly focused on a few epitopes within variant TS antigens appear to neither contribute to, nor detract from, the ability to control T. cruzi infection. These data also indicate that the relative position of an epitope within a CD8(+) immunodominance hierarchy does not predict its importance in pathogen control.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

