Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma
- PMID: 27354473
- PMCID: PMC5195913
- DOI: 10.1158/1078-0432.CCR-16-0870
Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma
Abstract
Purpose: Local tumor growth is a major cause of morbidity and mortality in nearly 30% of patients with pancreatic ductal adenocarcinoma (PDAC). Radiotherapy is commonly used for local disease control in PDAC, but its efficacy is limited. We studied the impact of selectively intervening on radiotherapy-induced inflammation as an approach to overcome resistance to radiotherapy in PDAC.
Experimental design: PDAC cell lines derived from primary pancreatic tumors arising spontaneously in KrasLSL-G12D/+;Trp53LSL-R172H/+;Pdx-1 Cre mice were implanted into syngeneic mice and tumors were focally irradiated using the Small Animal Radiation Research Platform (SARRP). We determined the impact of depleting T cells and Ly6C+ monocytes as well as inhibiting the chemokine CCL2 on radiotherapy efficacy. Tumors were analyzed by flow cytometry and IHC to detect changes in leukocyte infiltration, tumor viability, and vascularity. Assays were performed on tumor tissues to detect cytokines and gene expression.
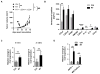
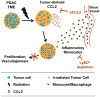
Results: Ablative radiotherapy alone had minimal impact on PDAC growth but led to a significant increase in CCL2 production by tumor cells and recruitment of Ly6C+CCR2+ monocytes. A neutralizing anti-CCL2 antibody selectively inhibited radiotherapy-dependent recruitment of monocytes/macrophages and delayed tumor growth but only in combination with radiotherapy (P < 0.001). This antitumor effect was associated with decreased tumor proliferation and vascularity. Genetic deletion of CCL2 in PDAC cells also improved radiotherapy efficacy.
Conclusions: PDAC responds to radiotherapy by producing CCL2, which recruits Ly6C+CCR2+ monocytes to support tumor proliferation and neovascularization after radiotherapy. Disrupting the CCL2-CCR2 axis in combination with radiotherapy holds promise for improving radiotherapy efficacy in PDAC. Clin Cancer Res; 23(1); 137-48. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
The authors have no competing financial interests.
Figures


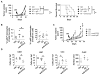
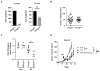
References
-
- Ryan D, Hong T, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–49. - PubMed
-
- American Cancer Society. Cancer Facts & Figures 2016. Atlanta: Am. Cancer Soc; 2016.
-
- Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541–6. - PubMed
-
- Moss AC, Morris E, Mac Mathuna P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst Rev. 2006:CD004200. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

