Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure
- PMID: 27354476
- PMCID: PMC5791838
- DOI: 10.1200/JCO.2016.67.3467
Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure
Abstract
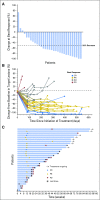
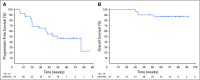
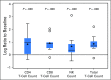
Purpose Classical Hodgkin lymphoma (HL) frequently exhibits genetic alterations leading to overexpression of the programmed death-1 (PD-1) ligands, suggesting a possible vulnerability to PD-1 blockade. The phase Ib study KEYNOTE-013 (NCT01953692) tested the safety and efficacy of the anti-PD-1 antibody pembrolizumab in patients with hematologic malignancies. Based on its genetics, HL was included as an independent cohort. Methods We enrolled patients with relapsed or refractory HL whose disease progressed on or after treatment with brentuximab vedotin. Patients received pembrolizumab, 10 mg/kg every 2 weeks, until disease progression occurred. Response to treatment was assessed at week 12 and every 8 weeks thereafter. Principal end points were safety and complete remission (CR) rate. Results Thirty-one patients were enrolled; 55% had more than four lines of prior therapy, and 71% had relapsed after autologous stem cell transplantation. Five patients (16%) experienced grade 3 drug-related adverse events (AEs); there were no grade 4 AEs or deaths related to treatment. The CR rate was 16% (90% CI, 7% to 31%). In addition, 48% of patients achieved a partial remission, for an overall response rate of 65% (90% CI, 48% to 79%). Most of the responses (70%) lasted longer than 24 weeks (range, 0.14+ to 74+ weeks), with a median follow-up of 17 months. The progression-free survival rate was 69% at 24 weeks and 46% at 52 weeks. Biomarker analyses demonstrated a high prevalence of PD-L1 and PD-L2 expression, treatment-induced expansion of T cells and natural killer cells, and activation of interferon-γ, T-cell receptor, and expanded immune-related signaling pathways. Conclusions Pembrolizumab was associated with a favorable safety profile. Pembrolizumab treatment induced favorable responses in a heavily pretreated patient cohort, justifying further studies.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Immunotherapy: High promise for pembrolizumab in HL.Nat Rev Clin Oncol. 2016 Jul 20;13(8):466. doi: 10.1038/nrclinonc.2016.114. Nat Rev Clin Oncol. 2016. PMID: 27435267 No abstract available.
-
Immune Therapies for Lymphomas: A Disruptive Technology With Opportunities for Radiation.Int J Radiat Oncol Biol Phys. 2018 Dec 1;102(5):1396-1399. doi: 10.1016/j.ijrobp.2018.05.079. Int J Radiat Oncol Biol Phys. 2018. PMID: 31014778 No abstract available.
References
-
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology. Hodgkin Lymphoma. Version 2.2015. Fort Washington, PA, National Comprehensive Cancer Network Inc., 2015. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

