Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer
- PMID: 27354478
- PMCID: PMC5019759
- DOI: 10.1200/JCO.2016.66.8822
Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer
Abstract
Purpose: Cisplatin is widely used but highly ototoxic. Effects of cumulative cisplatin dose on hearing loss have not been comprehensively evaluated in survivors of adult-onset cancer.
Patients and methods: Comprehensive audiological measures were conducted on 488 North American male germ cell tumor (GCT) survivors in relation to cumulative cisplatin dose, including audiograms (0.25 to 12 kHz), tests of middle ear function, and tinnitus. American Speech-Language-Hearing Association criteria defined hearing loss severity. The geometric mean of hearing thresholds (0.25 to 12 kHz) summarized overall hearing status consistent with audiometric guidelines. Patients were sorted into quartiles of hearing thresholds of age- and sex-matched controls.
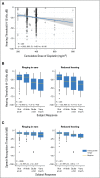
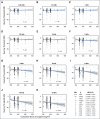
Results: Increasing cumulative cisplatin dose (median, 400 mg/m(2); range, 200 to 800 mg/m(2)) was significantly related to hearing loss at 4, 6, 8, 10, and 12 kHz (P trends, .021 to < .001): every 100 mg/m(2) increase resulted in a 3.2-dB impairment in age-adjusted overall hearing threshold (4 to 12 kHz; P < .001). Cumulative cisplatin doses > 300 mg/m(2) were associated with greater American Speech-Language-Hearing Association-defined hearing loss severity (odds ratio, 1.59; P = .0066) and worse normative-matched quartiles (odds ratio, 1.33; P = .093) compared with smaller doses. Almost one in five (18%) patients had severe to profound hearing loss. Tinnitus (40% patients) was significantly correlated with reduced hearing at each frequency (P < .001). Noise-induced damage (10% patients) was unaffected by cisplatin dose (P = .59). Hypertension was significantly related (P = .0066) to overall hearing threshold (4 to 12 kHz) in age- and cisplatin dose-adjusted analyses. Middle ear deficits occurred in 22.3% of patients but, as expected, were not related to cytotoxic drug dosage.
Conclusion: Follow-up of adult-onset cancer survivors given cisplatin should include routine inquiry for hearing status and tinnitus, referral to audiologists as clinically indicated, and hypertension control. Patients should be urged to avoid noise exposure, ototoxic drugs, and other factors that further damage hearing.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures




Comment in
-
The Sound of Silence: A Proxy for Platinum Toxicity.J Clin Oncol. 2016 Aug 10;34(23):2687-9. doi: 10.1200/JCO.2016.68.2476. Epub 2016 Jul 5. J Clin Oncol. 2016. PMID: 27382103 Free PMC article. No abstract available.
References
-
- Altekruse S, Kosary C, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD, National Cancer Institute, 2010.
-
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. - PubMed
-
- Schell MJ, McHaney VA, Green AA, et al. Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. J Clin Oncol. 1989;7:754–760. - PubMed
-
- Osanto S, Bukman A, Van Hoek F, et al. Long-term effects of chemotherapy in patients with testicular cancer. J Clin Oncol. 1992;10:574–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

