Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer
- PMID: 27354481
- PMCID: PMC5569693
- DOI: 10.1200/JCO.2016.66.9861
Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer
Abstract
Purpose: Nivolumab, a fully human immunoglobulin G4 programmed death-1 immune checkpoint inhibitor antibody, has demonstrated improved survival in previously treated patients with advanced non-small-cell lung cancer (NSCLC). CheckMate 012, a phase I, multicohort study, was conducted to explore the safety and efficacy of nivolumab as monotherapy or combined with current standard therapies in first-line advanced NSCLC. Here, we report results for nivolumab plus platinum-based doublet chemotherapy (PT-DC).
Patients and methods: Patients (N = 56) received nivolumab (intravenously) plus PT-DC concurrently every 3 weeks for four cycles followed by nivolumab alone until progression or unacceptable toxicity. Regimens were nivolumab 10 mg/kg plus gemcitabine-cisplatin (squamous) or pemetrexed-cisplatin (nonsquamous) or nivolumab 5 or 10 mg/kg plus paclitaxel-carboplatin (all histologies). The primary objective was to assess safety and tolerability. Secondary objectives included objective response rate and 24-week progression-free survival rate (per Response Evaluation Criteria in Solid Tumors version 1.1); exploratory objectives included overall survival (OS) and response by tumor programmed death ligand-1 expression.
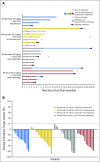
Results: No dose-limiting toxicities occurred during the first 6 weeks of treatment. Forty-five percent of patients (25 of 56 patients) reported grade 3 or 4 treatment-related adverse events (AEs); 7% of patients (n = 4) had pneumonitis. Twenty-one percent of patients (n = 12) discontinued all study therapy as a result of treatment-related AEs. Objective response rates for nivolumab 10 mg/kg plus gemcitabine-cisplatin, nivolumab 10 mg/kg plus pemetrexed-cisplatin, nivolumab 10 mg/kg plus paclitaxel-carboplatin, and nivolumab 5 mg/kg plus paclitaxel-carboplatin were 33%, 47%, 47%, and 43%, respectively; 24-week progression-free survival rates were 51%, 71%, 38%, and 51%, respectively; 2-year OS rates were 25%, 33%, 27%, and 62%, respectively. Responses were achieved regardless of tumor programmed death ligand-1 expression.
Conclusion: The safety profile of nivolumab plus PT-DC was consistent with that expected for individual agents; however, treatment discontinuation related to AEs was greater with the combination. Encouraging activity was observed, especially for the nivolumab 5 mg/kg plus paclitaxel-carboplatin group, with a 2-year OS rate of 62%.
Trial registration: ClinicalTrials.gov NCT01454102.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures


Comment in
-
Moving Programmed Death-1 Inhibitors to the Front Lines in Non-Small-Cell Lung Cancer.J Clin Oncol. 2016 Sep 1;34(25):2953-5. doi: 10.1200/JCO.2016.68.1205. Epub 2016 Jul 5. J Clin Oncol. 2016. PMID: 27382094 No abstract available.
-
Lung cancer: First-line immunotherapy in lung cancer - taking the first step.Nat Rev Clin Oncol. 2016 Oct;13(10):595-6. doi: 10.1038/nrclinonc.2016.148. Epub 2016 Sep 13. Nat Rev Clin Oncol. 2016. PMID: 27620712 No abstract available.
-
Efficacy and safety of nivolumab combined with standard therapies for first-line therapy of advanced non-small cell lung cancer.J Thorac Dis. 2016 Oct;8(10):E1254-E1256. doi: 10.21037/jtd.2016.10.26. J Thorac Dis. 2016. PMID: 27867601 Free PMC article. No abstract available.
References
-
- National Comprehensive Cancer Network: National Comprehensive Cancer Network Guidelines in Oncology. Non-Small Cell Lung Cancer Version 7.2015. www.nccn.org/professionals/physician_gls/f_guidelines.asp.
-
- Kelly K Crowley J Bunn PA Jr, etal: Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: A Southwest Oncology Group trial J Clin Oncol 19:3210–3218,2001 - PubMed
-
- Sandler A Gray R Perry MC, etal: Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer N Engl J Med 355:2542–2550,2006 - PubMed
-
- Scagliotti GV De Marinis F Rinaldi M, etal: Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer J Clin Oncol 20:4285–4291,2002 - PubMed
-
- Scagliotti GV Parikh P von Pawel J, etal: Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer J Clin Oncol 26:3543–3551,2008 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

