Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer
- PMID: 27354485
- PMCID: PMC5569692
- DOI: 10.1200/JCO.2016.66.9929
Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer
Abstract
Purpose: Nivolumab, a programmed death-1 (PD-1) immune checkpoint inhibitor antibody, has demonstrated improved survival over docetaxel in previously treated advanced non-small-cell lung cancer (NSCLC). First-line monotherapy with nivolumab for advanced NSCLC was evaluated in the phase I, multicohort, Checkmate 012 trial.
Methods: Fifty-two patients received nivolumab 3 mg/kg intravenously every 2 weeks until progression or unacceptable toxicity; postprogression treatment was permitted per protocol. The primary objective was to assess safety; secondary objectives included objective response rate (ORR) and 24-week progression-free survival (PFS) rate; overall survival (OS) was an exploratory end point.
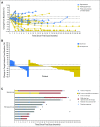
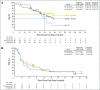
Results: Any-grade treatment-related adverse events (AEs) occurred in 71% of patients, most commonly: fatigue (29%), rash (19%), nausea (14%), diarrhea (12%), pruritus (12%), and arthralgia (10%). Ten patients (19%) reported grade 3 to 4 treatment-related AEs; grade 3 rash was the only grade 3 to 4 event occurring in more than one patient (n = 2; 4%). Six patients (12%) discontinued because of a treatment-related AE. The confirmed ORR was 23% (12 of 52), including four ongoing complete responses. Nine of 12 responses (75%) occurred by first tumor assessment (week 11); eight (67%) were ongoing (range, 5.3+ to 25.8+ months) at the time of data lock. ORR was 28% (nine of 32) in patients with any degree of tumor PD-ligand 1 expression and 14% (two of 14) in patients with no PD-ligand 1 expression. Median PFS was 3.6 months, and the 24-week PFS rate was 41% (95% CI, 27 to 54). Median OS was 19.4 months, and the 1-year and 18-month OS rates were 73% (95% CI, 59 to 83) and 57% (95% CI, 42 to 70), respectively.
Conclusion: First-line nivolumab monotherapy demonstrated a tolerable safety profile and durable responses in first-line advanced NSCLC.
Trial registration: ClinicalTrials.gov NCT01454102.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Moving Programmed Death-1 Inhibitors to the Front Lines in Non-Small-Cell Lung Cancer.J Clin Oncol. 2016 Sep 1;34(25):2953-5. doi: 10.1200/JCO.2016.68.1205. Epub 2016 Jul 5. J Clin Oncol. 2016. PMID: 27382094 No abstract available.
-
Lung cancer: First-line immunotherapy in lung cancer - taking the first step.Nat Rev Clin Oncol. 2016 Oct;13(10):595-6. doi: 10.1038/nrclinonc.2016.148. Epub 2016 Sep 13. Nat Rev Clin Oncol. 2016. PMID: 27620712 No abstract available.
References
-
- Kelly K Crowley J Bunn PA Jr, etal: Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non--small-cell lung cancer: A Southwest Oncology Group trial J Clin Oncol 19:3210–3218,2001 - PubMed
-
- National Comprehensive Cancer Network: NSCLC Guidelines v7. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl.
-
- Sandler A Gray R Perry MC, etal: Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer N Engl J Med 355:2542–2550,2006 - PubMed
-
- Scagliotti GV De Marinis F Rinaldi M, etal: Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer J Clin Oncol 20:4285–4291,2002 - PubMed
-
- Scagliotti GV Parikh P von Pawel J, etal: Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer J Clin Oncol 26:3543–3551,2008 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

