Conidial germination in Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans: influence of growth conditions and antifungal susceptibility profiles
- PMID: 27355215
- PMCID: PMC4957502
- DOI: 10.1590/0074-02760160200
Conidial germination in Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans: influence of growth conditions and antifungal susceptibility profiles
Abstract
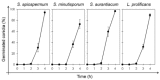
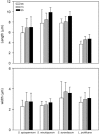
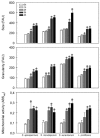
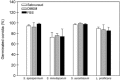
In the present study, we have investigated some growth conditions capable of inducing the conidial germination in Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans. Germination in Sabouraud medium (pH 7.0, 37ºC, 5% CO2) showed to be a typically time-dependent event, reaching ~75% in S. minutisporum and > 90% in S. apiospermum, S. aurantiacum and L. prolificans after 4 h. Similar germination rate was observed when conidia were incubated under different media and pHs. Contrarily, temperature and CO2 tension modulated the germination. The isotropic conidial growth (swelling) and germ tube-like projection were evidenced by microscopy and cytometry. Morphometric parameters augmented in a time-dependent fashion, evidencing changes in size and granularity of fungal cells compared with dormant 0 h conidia. In parallel, a clear increase in the mitochondrial activity was measured during the transformation of conidia-into-germinated conidia. Susceptibility profiles to itraconazole, fluconazole, voriconazole, amphotericin B and caspofungin varied regarding each morphotype and each fungal species. Overall, the minimal inhibitory concentrations for hyphae were higher than conidia and germinated conidia, except for caspofungin. Collectively, our study add new data about the conidia-into-hyphae transformation in Scedosporium and Lomentospora species, which is a relevant biological process of these molds directly connected to their antifungal resistance and pathogenicity mechanisms.
Figures




Similar articles
-
Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum and Lomentospora prolificans: a comparative study of surface molecules produced by conidial and germinated conidial cells.Mem Inst Oswaldo Cruz. 2018;113(6):e180102. doi: 10.1590/0074-02760180102. Epub 2018 Jun 18. Mem Inst Oswaldo Cruz. 2018. PMID: 29924142 Free PMC article.
-
N-Chlorotaurine Exhibits Fungicidal Activity against Therapy-Refractory Scedosporium Species and Lomentospora prolificans.Antimicrob Agents Chemother. 2015 Oct;59(10):6454-62. doi: 10.1128/AAC.00957-15. Epub 2015 Aug 3. Antimicrob Agents Chemother. 2015. PMID: 26239996 Free PMC article.
-
Assessment of biofilm formation by Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans.Biofouling. 2016 Aug;32(7):737-49. doi: 10.1080/08927014.2016.1192610. Biofouling. 2016. PMID: 27309801
-
Fusarium species,Scedosporium species, and Lomentospora prolificans: A systematic review to inform the World Health Organization priority list of fungal pathogens.Med Mycol. 2024 Jun 27;62(6):myad128. doi: 10.1093/mmy/myad128. Med Mycol. 2024. PMID: 38935914 Free PMC article.
-
Deep infections caused by Scedosporium prolificans. A report on 16 cases in Spain and a review of the literature. Scedosporium Prolificans Spanish Study Group.Medicine (Baltimore). 1997 Jul;76(4):256-65. doi: 10.1097/00005792-199707000-00004. Medicine (Baltimore). 1997. PMID: 9279332 Review.
Cited by
-
Mycobacterium ulcerans mycolactones-fungi crosstalking.Sci Rep. 2019 Feb 28;9(1):3028. doi: 10.1038/s41598-019-39927-3. Sci Rep. 2019. PMID: 30816261 Free PMC article.
-
A case of Scedosporium prolificans pulmonary infection in a patient with acute myeloid leukemia.Respir Med Case Rep. 2024 Jun 8;51:102071. doi: 10.1016/j.rmcr.2024.102071. eCollection 2024. Respir Med Case Rep. 2024. PMID: 38974754 Free PMC article.
-
Lomentospora prolificans: An Emerging Opportunistic Fungal Pathogen.Microorganisms. 2022 Jun 29;10(7):1317. doi: 10.3390/microorganisms10071317. Microorganisms. 2022. PMID: 35889036 Free PMC article. Review.
-
Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum and Lomentospora prolificans: a comparative study of surface molecules produced by conidial and germinated conidial cells.Mem Inst Oswaldo Cruz. 2018;113(6):e180102. doi: 10.1590/0074-02760180102. Epub 2018 Jun 18. Mem Inst Oswaldo Cruz. 2018. PMID: 29924142 Free PMC article.
-
Varying susceptibility of clinical and environmental Scedosporium isolates to chemical oxidative stress in conidial germination.Arch Microbiol. 2018 Apr;200(3):517-523. doi: 10.1007/s00203-018-1491-5. Epub 2018 Feb 20. Arch Microbiol. 2018. PMID: 29464281
References
-
- Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460(7259):1117–1121. - PubMed
-
- Allen PJ. Metabolic aspects of spore germination in fungi. Annu Rev Phytopathol. 1965;3:313–342.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

